The Act on the Registration and Evaluation of Chemicals (known as Korea REACH) passed the plenary session of the National Assembly in Korea on April 30th, 2013 and came into force on January 1st, 2015. In December 2016, the Ministry of Environment (MoE) started to revise the K-REACH and the amended K-REACH came into force on the 1st of January 2019.
The purpose of K-REACH is to protect public health and the environment through these provisions:
Registration and Notification of Chemical Substances
Exemption from Registration or Notification of Chemical Substances
Notification of Substances Subject to Priority Control Chemical Substances & Notification of Products Containing Priority Control Chemical Substances
Information Provision
The Ministry of Environment (MoE) is responsible for the registration and evaluation of chemical substances under this Act.
News Updates
In March 2024, the South Korean government released the latest government support program for small and medium-sized enterprises, to support the K-REACH registration and reduce the cost burdens.
According to Korea REACH, we have summarized some key dates as follows:
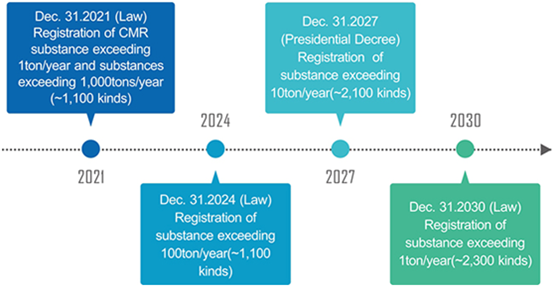
31st December 2030 | Deadline for registration of substances exceeding 1t/y. |
|---|---|
31st December 2027 | Deadline for registration of substances exceeding 10t/y |
31st December 2024 | Deadline for registration of substances exceeding 100t/y |
31st December 2021 | Deadline for registration of CMR substances exceeding 1t/y and substances exceeding 1000t/y |
30th June 2019 | Deadline for K-REACH pre-registration |
1st January 2019 | Commencement of the amended K-REACH |
1st July 2018 | End of grace period - PECs joint registration |
1st July 2015 | Release the 1st batch of PECs substances subject to registration under K-REACH |
1st January 2015 | "K-REACH" comes into force |
Scope of K-REACH
Korea REACH does not apply to:
① Radioactive materials defined in subparagraph 5 of Article 2 of the Nuclear Safety Act.
② Drugs defined in subparagraph 4 of Article 2 of the Pharmaceutical Affairs Act and quasi-drugs defined in subparagraph 7 of the said Article.
③ Narcotics defined in subparagraph 1 of Article 2 of the Narcotics Control Act.
④ Cosmetics defined in subparagraph 1 of Article 2 of the Cosmetics Act and raw materials used for cosmetics.
⑤ Pesticides defined in subparagraph 1 of Article 2 of the Pesticide Control Act and active ingredients defined in subparagraph 3 of the said Article.
⑥ Fertilizers defined in subparagraph 1 of Article 2 of the Fertilizer Control Act.
⑦ Foods defined in subparagraph 1 of Article 2 of the Food Sanitation Act, food additives defined in subparagraph 2 of the said Article, apparatus defined in subparagraph 4 of the said Article, containers and packages defined in subparagraph 5 of the said Article.
⑧ Feed defined in subparagraph 1 of Article 2 of the Control of Livestock and Fish Feed Act.
⑨ Explosives defined in Article 2 (3) of the Act on Control of Guns, Swords, and Explosives.
⑩ Munitions defined in Article 2 of the Act on the Management of Military Supplies and subparagraph 2 of Article 3 of the Defence Acquisition Program Act (excluding ordinary commodities prescribed in Article 3 of the Act on the Management of Military Supplies).
⑪ Health functional foods defined in subparagraph 1 of Article 3 of the Health Functional Foods Act.
⑫ Medical devices defined in Article 2 (1) of the Medical Devices Act.
⑬ Hygiene products defined in subparagraph 1 of Article 2 of the Hygiene Products Control Act.
⑭ Biocidal substances and biocidal products defined in subparagraphs 7 and 8 of Article 3 of the Act on Safety Control of Consumer Chemical Products and Biocides.
Overview of K-REACH
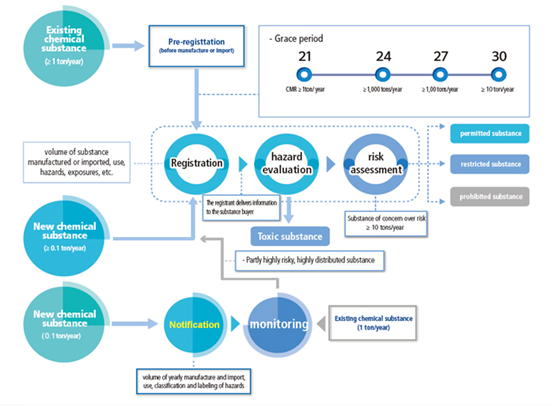
Pre-registration
In accordance with the requirements of the amended K-REACH, manufacturers/importers that manufactured or imported at least one ton per year of an existing substance between 2016 and 2018, shall submit application for pre-registration between the 1st of January 2019 and the 30th of June 2019. Enterprises that have completed the pre-registration can enjoy a corresponding registration grace period based on the tonnage band as well as the hazards of substances.
Enterprises that have not completed pre-registration or registration cannot continue to manufacture/import/export substances exceeding 1 ton/year after the 1st of July 2019. If related enterprises illegally manufacture/import/use substances, the responsible enterprise or responsible person may be imprisoned for no more than 5 years or receive a fine not exceeding 100,000,000 won, depending on the number of illegal activities and the seriousness of violations.
Registration
Enterprises that intend to manufacture or import the following substances are obligated to complete K-REACH registration:
At least 0.1 ton/year of a new chemical substance;
Existing substances manufactured, imported or sold in quantities greater than 1 ton per year;
New substances manufactured or imported in quantities greater than 0.1 ton/year shall be registered prior to manufacture or importation. A grace period (given to pre-registered substances) will be granted to existing substances to be manufactured or imported in quantities greater than 1 ton/year.
Similar to EU REACH, foreign manufacturers exporting chemical substances or products containing hazardous chemical substance into South Korea may appoint an Only Representative to fulfil relevant obligations under K-REACH.
Definition of New Substances
A new substance is defined as a substance that is not on the Korea Existing Chemicals List (KECL), which includes:
Chemical substances which were placed on the Korean market before the 2nd of February, 1991, and notified by the Ministry of Environment on the 23rd of December, 1996; and
Chemical substances that have undergone the examination of toxicity under the former provisions or the provisions of this Act after 2nd of February, 1991 and were announced by the Minister of Environment.
Information Required for Registration
The following information is required for registration:
The name and address of a manufacturer or an importer or an only representative.
Information that identifies a chemical substance including its name, molecular formula and graphic formula.
Identified uses of the chemical substance.
Classification and labelling of the chemical substance.
Physical and chemical properties.
Hazard data (toxicology/eco-toxicology data).
Risk associated with the chemical substance including exposure scenarios describing how to handle and control the substance (Applicable only when the substance is manufactured or imported in 10 tons or more per year).
Guidance on safe uses(including protective equipments, response to an explosion, a fire or a leak).
Other information specified in the Environment Ministerial Decree .
Registration - Joint Submission
Enterprises that intend to register existing chemical substances within the registration grace period shall individually apply for registration, but in cases of the data for application for registration. However, in relation to the data required for the registration such as test data for hazardous chemical substances, they shall jointly submit such data by forming a consortium. The procedures of joint registration are as follows:

Registration - Polymer Registration
Polymer registration requires less data on the properties of the polymer, e.g. acid/base stability test, and residual monomer contents test than normal (non-polymer) registration.
In accordance with the paragraph 3 of article 2 of the K-REACH Enforcement decree, a polymer is defined as a substance meeting the following criteria:
It shall be composed of molecules in which at least one kind of monomer unit is continuously repeated.
It shall show the characteristic distribution of molecular weights in accordance with repetitive numbers of monomer units in each molecule.
Its molecules that at least three monomer units form a covalent bond with at least one monomer unit or other reactants shall be at least 50 percent.
Its molecules of the same molecular weight shall be not more than 50 percent of the weight ratio.
Chemical Substances Exempted from Registration or Notification
Substances falling under any of the following subparagraphs are subject to exemption from registration:
A chemical substance imported as contained in machinery.
A chemical substance imported along with machinery or equipment for a test run.
A chemical substance in a product in solid state with specific shape for a certain function and not discharged during its use.
A chemical substance designated and publicly notified by the Minister of Environment after deliberation by the Chemical Substance Evaluation Committee which has extremely low risk.
Impurities, by-products, minerals, ores, glass, vegetable fats/oil, hydrogen, and oxygen, etc.
A substance itself existing in the nature or a substance obtained by using manpower, machine or gravity to the substance existing in the nature or by selecting after dissolving or floating thereof in the water.
A base composing DNA or RNA, nucleoside which is a combination of base and sugar.
The following substances will also be exempted from registration or notification after obtaining confirmation of exemption from registration. Application for confirmation of exemption from registration shall be made via the Chemical Substance Information Processing System before manufacturing or importation of the substance:
Reagents for test, research or inspection.
A chemical substance for demo production, such application may be submitted within 30 days from the date such substance has been manufactured or imported (within 14 days until the 31st of December, 2018).
Information Provision
Anyone who transfers a registered chemical substance or preparation containing the substance shall provide the following information to downstream users:
Name or trade name, location and phone number of the provider of the chemical substance safety information.
Name of the product and name or generic name of the relevant chemical substance.
Registration number or report number and serial number of the relevant chemical substance.
Classification and labelling of the relevant chemical substance.
The uses available for use or the uses restricted for use of the relevant chemical substance.
Physical and chemical properties of the relevant chemical substance and the information relating to human bodies and environmental hazards.
Summarized information of the exposure scenario and the risk information such as measures to reduce the risks.
Information concerning safe use of the relevant chemical substance such as operational methods of the relevant chemical substance, measures in emergency such as fire, manner of control at the time of leaking, protective equipment and method of disposal.
Regulatory information for the relevant chemical substance.
Notification of Substances subject to Intensive Control & Notification of Products Containing Intensive Control Chemicals
Enterprises that manufacture or import any product containing any substance of intensive control shall notify the Minister of Environment before manufacturing or importation if the both of the following conditions apply to the product:
1) The weight of the individual substance of priority control in the product exceeds 0.1 weight percent.
2) The gross weight of the individual substances of priority control contained in the total products exceeds one ton per year.
Substances falling under any of the following are substances of priority control:
A substance which may be or is concerned to be carcinogenic, mutagenic, toxic for reproduction, or disruptive to endocrine system to human beings or animals.
A substance which is easily accumulated in the bodies of human beings, animals or plants and remains in the environment for a long period of time.
A substance which may damage organs such as the lungs, livers and kidneys of human beings if human beings are exposed for a long period of time or repeatedly to the substance.
A substance which may give the same level or higher level of serious risk to human beings, animals or plants compared with the above substances.
Our Korea Chemical Compliance Services
K-REACH Only Representative.
K-REACH (late) Pre-registration.
K-REACH Registration.
K-REACH Lead Registrant Service.
New Substance Inquiry.
K-REACH Registration Exemption Application.
Chemical safety Report.
Technical Support.
Preparation of Korean SDS and label.