There are many regulations relating to cosmetic labeling in China. In this article, we will provide some details of what cannot be contained on the labels and some examples of improper labeling.
Major regulations and requirements regarding cosmetic labeling in China:
- GB 5296.3-2008 Instruction for Use of Consumer Products-General Labeling for Cosmetics
- Provisions on the Management of Cosmetics Labeling
- Measures for the Supervision and Administration of Management on Prepackaged Products
- Notice on Printing and Distributing the Naming Regulations and Guidelines for Cosmetics
- Management Requirements for Sun Protection Effect Label of Sun Protection Cosmetics
- Measures for Administration of Cosmetics Labeling
According to Article 19 of the Measures for Administration of Cosmetics Labeling, cosmetic labeling must not be marked or declared in the following ways:
- Use medical terminologies, names of medical celebrities, words describing medical effects, or the names of approved drugs indicating or implying the medical effects of the products;
- Use false, exaggerated, and absolute words to make false or misleading descriptions;
- Use trademarks, designs, font color, size, color difference, homophonic or suggestive words, letters, Chinese Pinyin, numbers, or symbols to imply the medical effects or make false claims;
- Mislead consumers by using terms and mechanisms that have not been widely accepted by the scientific community;
- Mislead consumers by fabricating false information and belittling other legitimate products;
- Mislead consumers by using fictitious, forged, or unverifiable scientific research achievements, statistical data, survey results, abstracts, quotations, and other information;
- Claim the functions of cosmetic ingredients to imply functions of products that the products do not actually have or are not allowed to claim;
- Use marks and terms that have not been confirmed by competent authorities to promote the safety and efficacy claims of cosmetics;
- Make use of the name and image of state organs, institutions, medical institutions, public welfare institutions, and other units and their staff, and employed experts to prove or recommend;
- Assertions or guarantees of efficacy and safety;
- Mark vulgar, feudal superstition or other content that violates public order and good customs; and
- Other contents prohibited by laws, administrative regulations, and mandatory national standards for cosmetics.
Relevant regulations and requirements on cosmetics labelings in other countries and regions:
The European Union | Regulation (EC) No. 1223/2009 |
America | Cosmetic Labeling Guide |
Japan | Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices |
South Korea | Cosmetics Act |
Canada | Industry Guide for the Labeling of Cosmetics |
Taiwan, China | General Instructions on the Marking Regulations of Cosmetic Packaging, Containers, Labels or Copy Sheets |
ASEAN (the Association of Southeast Asian Nations) | ASEAN Cosmetic Directive, ASEAN Sunscreen Labeling Guideline |
Improper Cosmetic Labeling Cases
The following are seven cases of improper cosmetic labeling on cosmetic packages and each represents one situation.
(1)

Comments: Cosmetics refer to daily use chemical industrial products that can be applied to skin, hair, nails, lips, and other human surfaces by rubbing, spraying, or other similar methods for the purpose of cleaning, protecting, beautifying, and modifying. “...reactive its natural structures and processes” is beyond the definition of cosmetics.
(2)

Comments: The cosmetic product is “designed for women and men of all age groups and skin types”. However, for users aged 0-12, cosmetics should be registered according to the requirements for children’s cosmetics.
(3)

Comments: According to Article 19 (3) of Measures for Administration of Cosmetics Labeling, it is prohibited to use trademarks, designs, font color, size, color difference, homophonic or suggestive words, letters, Chinese Pinyin, numbers, symbols, etc. to imply the medical effects or make false claims;
The red-cross sign in the package is usually associated with medical functions such as first aid and hospitals. The use of red-cross may imply a medical effect of the product. But there is one exception-the product is produced in Switzerland. The Swiss flag logo which will not mislead consumers can be marked to indicate the place of production.
(4)
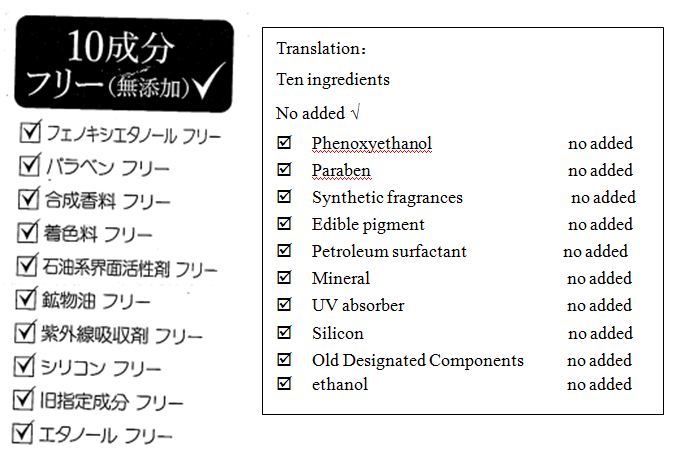
Comments: Suspected of belittling other legitimate products and misleading consumers.
(5)
 5.
5.
Comments: The product is anti-wrinkle, moisturizing, and cleansing foam, which belongs to rinse-off products. According to Inspection Work Standards for the Registration and Filing of Cosmetics, the safety evaluation of human trial tests should be carried out for the rinse-off products that claim the efficacy of acne elimination, wrinkle resistance, and spot removal.
(6)

Comments: ①If the product uses stickers and membrane carrier materials, the source, preparation process, quality control indicators, and other data of the carrier must be provided, and the material composition of the carrier shall be noted; ②Words such as “powerful” and “effective” cannot be clearly determined, and are easy to mislead consumers, so it is not recommended to declare; ③ “Quickly fill the skin” and “eliminate fine lines” are exaggerated claims.
(7)

Comments: ①“ANTI-POLLUTION” is newly added. It is suggested to get registered according to the requirements of new efficacy products. ②“ANTI-REDNESS” is a medical term and it goes beyond the definition of cosmetics.
If you have any needs or questions, please contact us at service@cirs-group.com.

