On 17 Jan, 2017, CFDA and AQSIQ jointly issued a Notice regarding Pilot Implementation of Record-keeping for Imported Non-special Use Cosmetics in Shanghai Pudong New Area (Notice No.7.2017). It means from 1 Mar, 2017 to 21 Dec, 2018, the regulatory regime of first imported non-special use cosmetics in Shanghai Pudong New Area will change to record-keeping from current registration. On 18 Jan, 2017, CFDA issued a Notice on Work Program of Record-keeping for Imported Non-special Use Cosmetics in Pudong New Area (Temporary, Notice No.10, 2017). The document covers the main procedures of record-keeping and documents requirements.
Background
On 19 Apr., 2016, the State Council issued a Decision regarding Temporary Adjustment of Administrative Regulations and Approval Matters Stipulated on State Council Documents in Shanghai Pudong New Area (State Council No. 24, 2016), pointing out that there will be 11 temporarily adjusted administrative regulations and approval matters stipulated on State Council Documents from now until 21 Dec., 2018 in Shanghai Pudong New Area. This decision involves 6 issues, such as drug advertising, radioactive drugs, cosmetics, etc.
Flowchart of regulatory compliance for the first import of non-special use cosmetics in difference cases in 2017
Case 1: the products are imported from the Shanghai Pudong New Area, and the responsible person is registered in Pudong New Area (Shanghai FDA record-keeping)
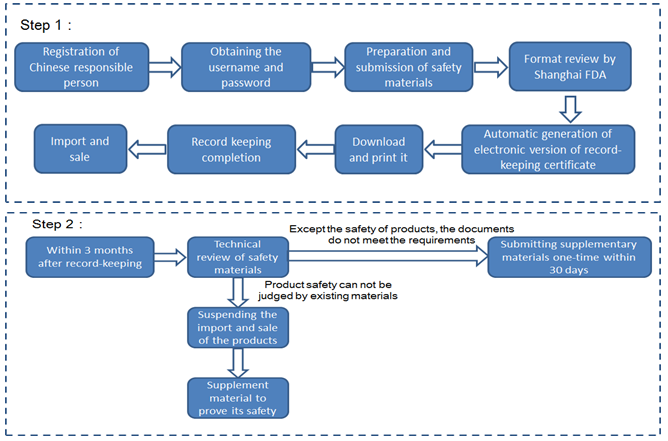
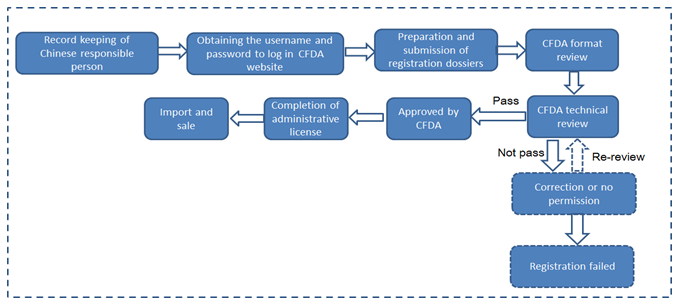
Case 2: the products are imported from any other ports except Pudong New Area (CFDA registration)
Case 3: the products recorded in Shanghai FDA are no longer to be imported from Pudong New Area

Case 4: the products are imported from Pudong New Area and other ports.
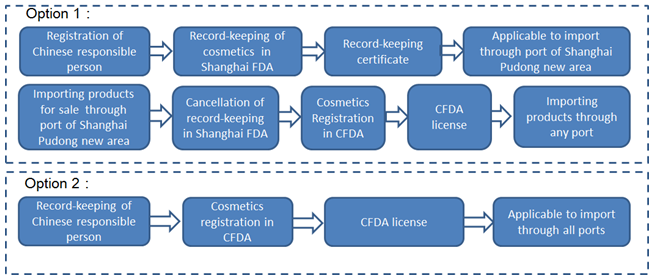
It can be seen that there are different options for enterprises to choose the pre-market record-keeping or registration of imported non special use cosmetics based on actual situations. Meanwhile it is the same for special use cosmetics to be approved by CFDA. The following table summarizes the different ways of record-keeping or registration in terms of type of products, the port of entry, and the characteristics of enterprises.
Product Type | Import Port | Registering a company in Pudong or not | Sales model | Market access program |
Imported non-special use cosmetics | Pudong New Area | YES | Offline | Record-keeping in Shanghai FDA |
Imported non-special use cosmetics | Pudong New Area and others | NO | Offline | Registration in CFDA |
Imported non-special use cosmetics | Pudong New Area and others | YES | Offline | Option 1: Record-keeping in Shanghai FDA at first. Then cancelling the record-keeping in Shanghai FDA before CFDA registration. Finally applying for CFDA registration. Option 2: Registration in CFDA |
Imported special use cosmetics | - | - | Offline | Registration in CFDA |
Domestic special use cosmetics | - | - | Offline | Registration in CFDA |
Domestic non special use cosmetics | - | - | Offline | Record-keeping in local FDA |
Import cosmetic | - | - | Online | CFDA registration is not required for 10 pilot cities such as Tianjin, Shanghai, Hangzhou, Ningbo, Zhengzhou, Guangzhou, Shenzhen, Chongqin, Fuzhou, Pingtan etc before 31 Dec 2017. |
Comparisons of the two ways of importing non special use cosmetics to China
Item | Registration in CFDA | Record-keeping in Shanghai FDA |
Competent department | CFDA | Shanghai FDA |
Authorized application | Chinese responsible person | Chinese responsible person |
Requirements of responsible person | No restriction except legal entity | Registered in Pudong New Area |
Responsibilities of responsible person | Application of administrative license | Import and operation, product quality and safety |
Application scope | All the ports of the country | Pudong New Area in Shanghai |
Required documents | Almost the same | |
Format review organization | Acceptance Center of CFDA | Shanghai FDA |
Technical review | After the formal review, transferring to CFDA Health Food Review Center for technical review | Within 3 months after record-keeping |
Issue of CFDA license or Record-keeping certificate | Issued after passing both format and technical review | Automatically generating electronic version of record number after format review for the enterprise to download and print |
Format of license or certificate number | 国妆备进字J+ year number +4 digits | 国妆网备进字(沪)+ year number +6 digits |
Term of validity | 4 years | Undetermined |
No longer importing recorded or registered products | No requirements | Cancellation of record-keeping information |
CIRS comments
- As is shown in the table, the biggest difference between CFDA registration and the record-keeping in Shanghai FDA is the process and the requirements of the responsible person.
- The biggest advantage of record-keeping in Shanghai FDA is that the products can be imported for sale after format review, while CFDA requests to pass the format and technical review before the issuance of CFDA license. This is good news for enterprise in demands of rapid time-to-market.
- The documents for CFDA registration or Shanghai record-keeping are almost the same.
- More responsibilities are required to the responsible person for the record-keeping in Shanghai FDA compared with the CFDA registration. The responsible person registered in Pudong New Area shall bear the responsibility of product quality and safety.
If you have any questions, please contact us at service@cirs-group.com.

