On January 13, 2025, the Scientific Committee on Consumer Safety (SCCS) of the European Union issued preliminary opinion (SCCS/1674/25) on Butylparaben (CAS No. 94-26-8). The deadline for comments is March 10, 2025.

The SCCS concludes the following:
Based on the safety assessment carried out in consideration of all available information, including the potential endocrine effects, the SCCS is of the opinion that the use of Butylparaben as a preservative at a maximum concentration of 0.14 % (as acid) in all cosmetic products included in this exposure assessment is not safe for children between 0.5-1 years, 1-3 years, 3-6 years and 6-10 years when used in combination. With the exception of body lotion, it is safe in single dermal and oral product categories, when used only in the respective product category.
In the SCCS’s opinion, to be safe for all the childrens’ age groups that were considered, the maximum concentration of Butylparaben in final products should not exceed 0.028% (as acid). However, the necessity of a reduction in Butylparaben concentration could be reassessed when a well-carried out dermal absorption study and better exposure studies specifically tailored to EU children become available.
This Opinion is not applicable to any sprayable product (including mouth spray) that may lead to exposure of end-user’s lungs by inhalation. The SCCS mandates do not address environmental aspects. Therefore, this assessment did not cover the safety of Butylparaben for the environment.
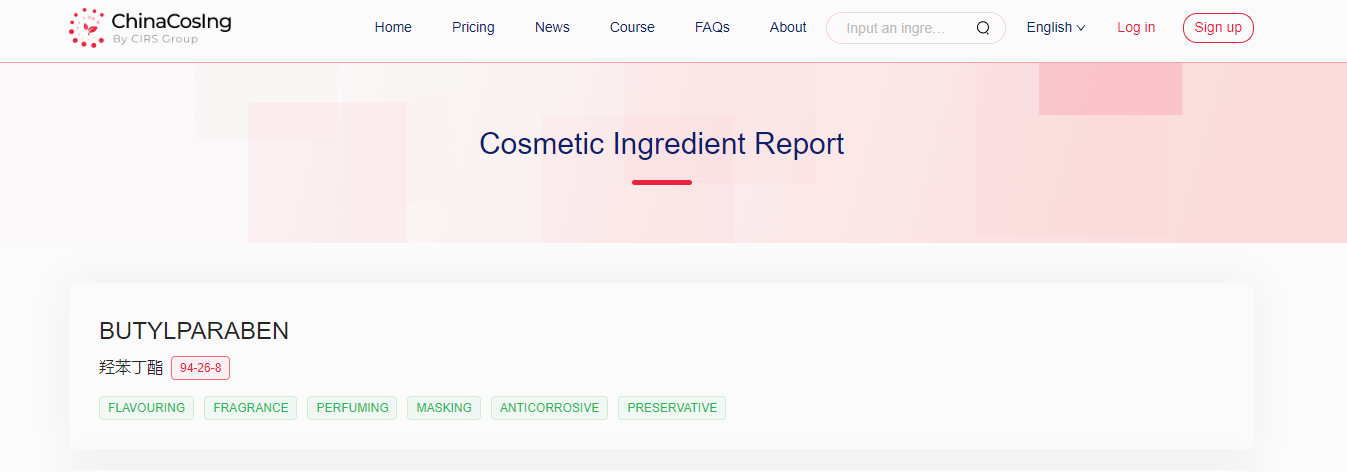
The Chinese Cosmetic Ingredient Regulatory Database (ChinaCosIng), independently developed by CIRS Group, indicates that Butylparaben (CAS No. 94-26-8), used as flavoring, fragrance, perfuming, masking, anticorrosive and preservative in cosmetics.
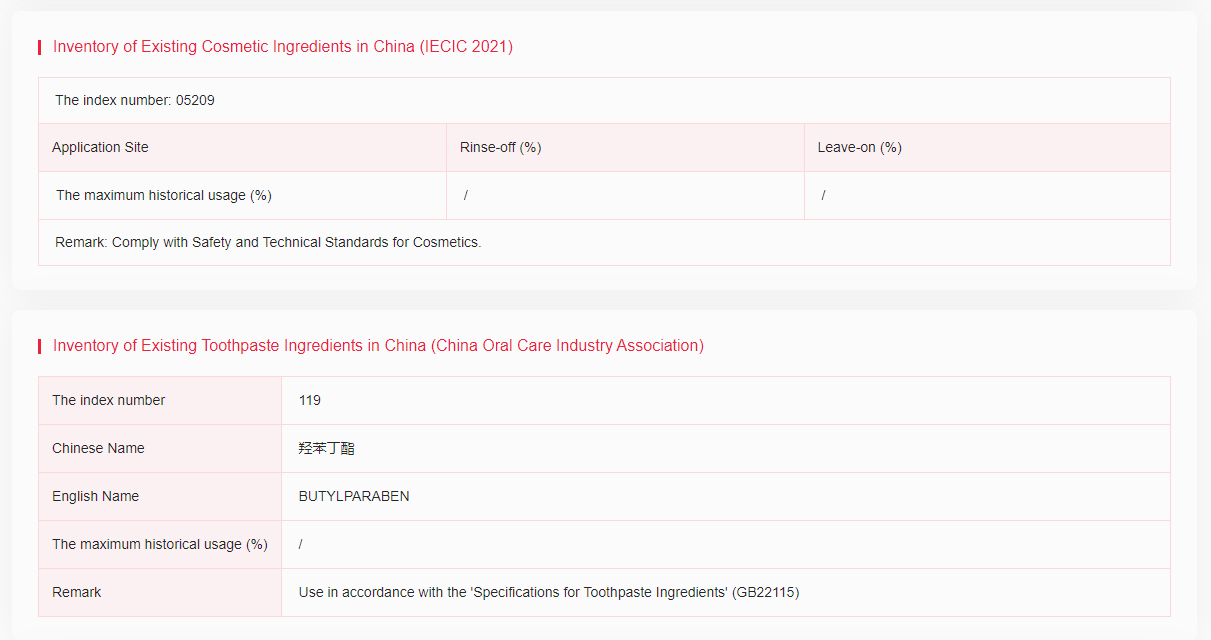
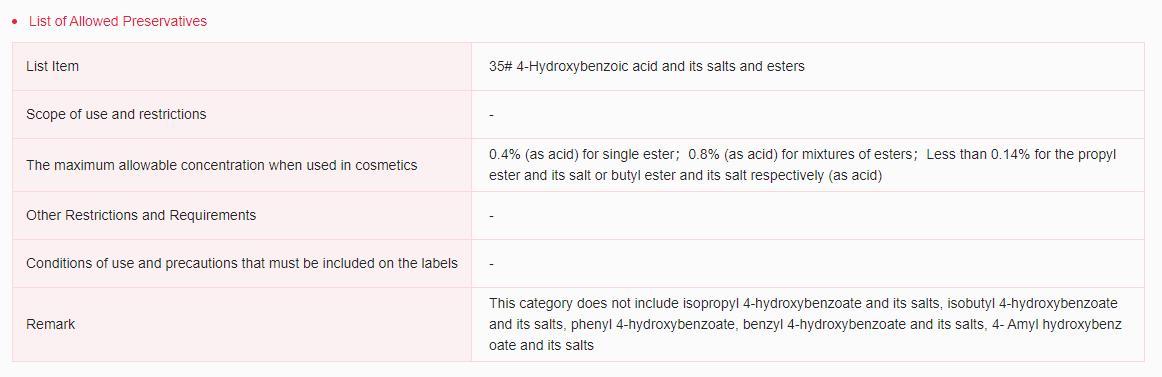
It has been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC) (2021 edition), Inventory of Existing Toothpaste Ingredients in China (China Oral Care Industry Association) and List of Allowed Preservatives. When used as preservatives in cosmetics, the maximum allowable concentration is 0.4% (as acid) for single ester; 0.8% (as acid) for mixtures of esters; less than 0.14% for the propyl ester and its salt or butyl ester and its salt respectively (as acid).
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

