On September 15, 2022, EU released the Commission Regulation 2022/2031 as regards the prohibitions and restrictions on substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR) and also updated Annex II, III and V to Regulation (EC) No 1223/2009. The amended Regulation shall enter into force on the twentieth day after its publication, and the restrictions on CMR shall be applied from December 17, 2022.
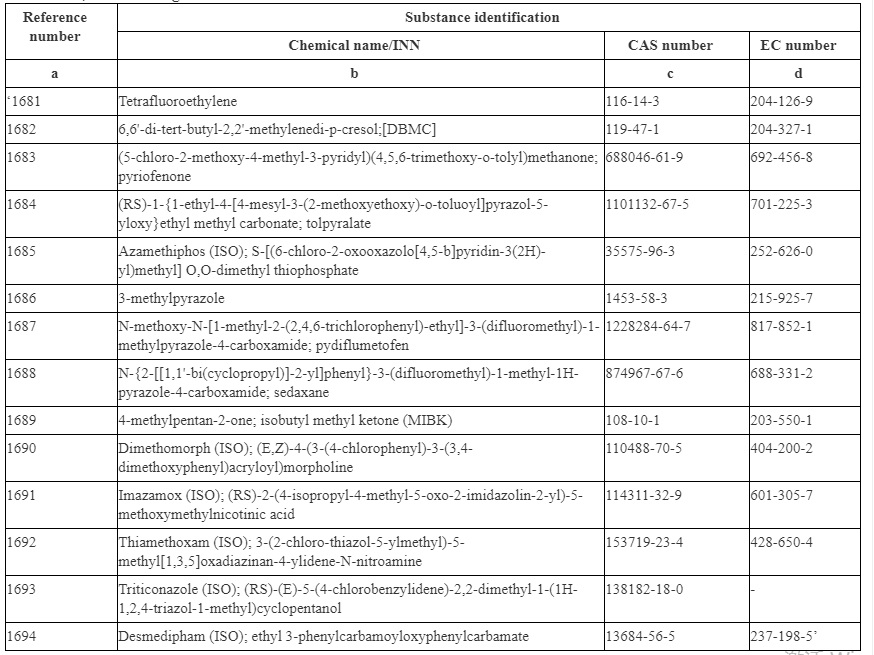
The following substances are listed in Annex II as they are classified as CMR Category 1A, 1B or Category 2. These substances include Tetrafluoroethylene, 4-Methyl-2-pentanone (MIBK) and some pesticides.
There is one exemption. Methyl 2-hydroxybenzoate (CAS No: 119-36-8) is classified as CMR Category 2 (Toxic for Reproduction), but Scientific Committee on Consumer Safety (SCCS) believed that it is safe for use in cosmetics when the safe use concentration is not exceeded. The safe use concentration for Methyl 2-hydroxybenzoate is amended as following:
- Leave-on skin products (except face makeup, spray/aerosol body lotion, spray aerosol deodorant and hydroalcoholic-based fragrances) and leave-on hair products (except spray/aerosol products): maximum concentration is 0.06%;
- Face makeup (except lip products, eye makeup and makeup remover): maximum concentration is 0.05%;
- Eye makeup and makeup remover: maximum concentration is 0.02%,
- Leave-on hair products (spray/aerosol): maximum concentration is 0.009%;
- Deodorant spray/aerosol: maximum concentration is 0.003%;
- Body lotion spray/aerosol: maximum concentration is 0.04%;
- Rinse-off skin products (except hand wash) and rinse-off hair products: maximum concentration is 0.06%;
- Hand wash: maximum concentration is 0.6%;
- Hydroalcoholic-based fragrances: maximum concentration is 0.6%;
- Lip products: maximum concentration is 0.03%;
- Toothpaste: maximum concentration is 2.52%;
- Mouthwash intended for children aged 6-10 years of age and adults: maximum concentration is 0.1%;
- Mouthwash intended for children above 10 years of age and adults: maximum concentration is 0.6%; and
- Mouth spray: maximum concentration is 0.65%.
In Annex V, Sodium N-(hydroxymethyl) glycinate (CAS Number: 70161-44-3) is corrected as follows: Not to be used unless it can be shown that the maximum theoretical concentration of releasable formaldehyde, irrespective of the source, in the mixture as placed on the market is < 0,1 % w/w2 whose maximum concentration is 0.5%.
Methyl 2-hydroxybenzoate and Sodium hydroxymethyl glycinate are both listed in the Inventory Existing Cosmetics Ingredients China (IECIC). If you would like to know more information, please refer to the Chinese Cosmetic Ingredient Regulatory Databse (China CosIng).
If you have any needs or questions, please contact us at service@cirs-group.com.
Further Information
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R1531&qid=1663560995832

