A New Dietary Ingredient (NDI) is defined in 21 CFR as dietary ingredients, ingredients used as supplements, introduced to the US market after October 15, 1994. As required by the FDA, manufacturers must notify the FDA at least 75 days prior to the marketing of a product containing any NDI. Almost all NDI notifications (NDIN) and their corresponding FDA response letters are available to the public on the FDA website. The acceptance of the information provided in each NDIN is a procedural matter that does not preclude the FDA from taking action against the NDI if it is found to be unsafe in the future. NDIN submission statistics are summarized below.
NDIN submissions since 2021
Since 2021, a total of 136 NDINs have been submitted (Figure 1) among which, 79 were accepted and 42 were rejected (The reasons for rejection are stated in the FDA’s response letter), and 12 with no response as the FDA were not able to identify the notified substances as dietary ingredients (Figure 2 and Figure 3).
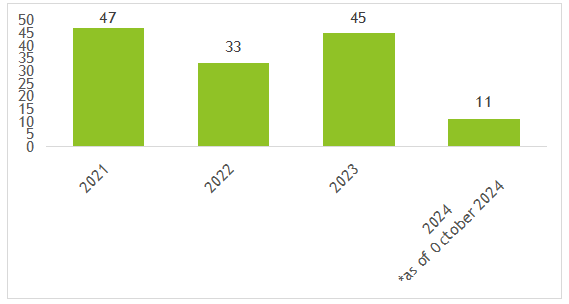
Figure 1. The number of NDINs submitted to the FDA each year since 2021
Data is updated as of October 2024
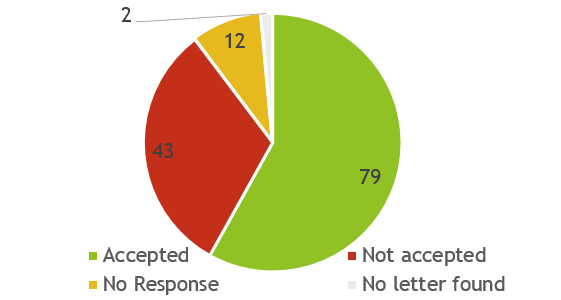
Figure 2. Statistics of the FDA responses to each submission since 2021
Data is updated as of October 2024
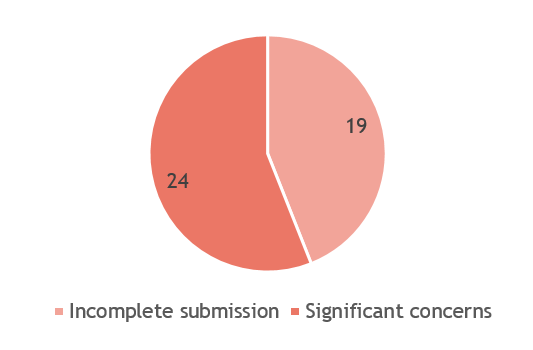
Figure 3. Reasons for rejected submission
Data is updated as of October 2024
Resubmissions
Approximately a fifth of those have been accepted after they have been resubmitted (See Figure 4). Generally, most resubmissions could be accepted in the second time. But NDIN #1306 is an exception. It was only accepted after the fourth submission.
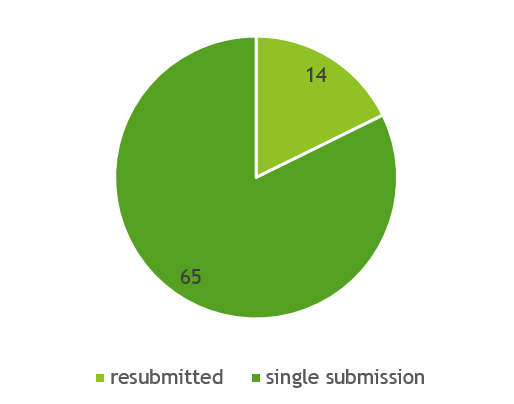
Figure 4. The number of accepted NDINs are from a single submission or from a resubmission of a previously rejected submission.
Data is updated as of October 2024
Nicotinamide mononucleotide (NMN): A Special case
Among those with no response from the FDA, as they could not be identified as dietary ingredients with the information provided in the notification, several were Nicotinamide mononucleotide (NMN). Previously, the FDA approved β-NMN submitted by Syncozyme (Shanghai) after the second submission (NDID #1247) on May 16, 2022. However, this was withdrawn on November 4, 2022, in a supplemental response letter.
Then on March 6, 2023, the National Products Association (NPA) filed a citizen petition to the FDA asking the FDA to reconsider their view on NMN as an NDI. The FDA replied in a Notification that their response to the notification will depend on their decision on this petition. On August 30, 2023, the FDA replied to this citizen petition saying that “due to competing agency priorities”, they have not come to a decision regarding the status NMN.
A year later on August 28, 2024, NPA filed a lawsuit against the FDA “requesting that the agency cease its unlawful retroactive application of the Food, Drug, and Cosmetic Act against Nicotinamide Mononucleotide (NMN)”. Then on October 24, 2024, the FDA requested a stay of the legal proceedings to the US District Court for the District of Columbia in regards to this lawsuit while they evaluated the concerns of the NPA’s petition. The FDA stated that they will provide a conclusion to the approval of NMN by July 31, 2025. As they assessed the citizen petition, the FDA presented to the NPA that they would not prioritize enforcement actions against the marketing of NMN as an ingredient in dietary supplements, provided that they would be lawful if NMN is not excluded from the definition of being a “dietary supplement”.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

