According to Food Safety Law, China food category can be divided into two categories: General food and Special food. Under the category of special food, there are health food, infant formula food and food for special medical purposes (FSMP). Among them, infant formula food is easy to be distinguished from other foods because of its obvious characteristics. However, health food, FSMP as well as general food are often confused by the public. CIRS has summarized the differences of these three types of food from 7 aspects, and added the drug to make comparison, to help the public distinguish these product categories correctly.
1. Definition
Definition | |
General food | Food products and food raw materials that used for human consumption. General food can be eaten by all people, and provide energy and nutrients. |
Health food | I. Food products with health function claims based on scientific basis, and have no acute, sub-acute or chronic hazards to human body. II. In Chinese industry, health food is usually divided into two categories: 1) nutrition supplement; 2) functional health food. |
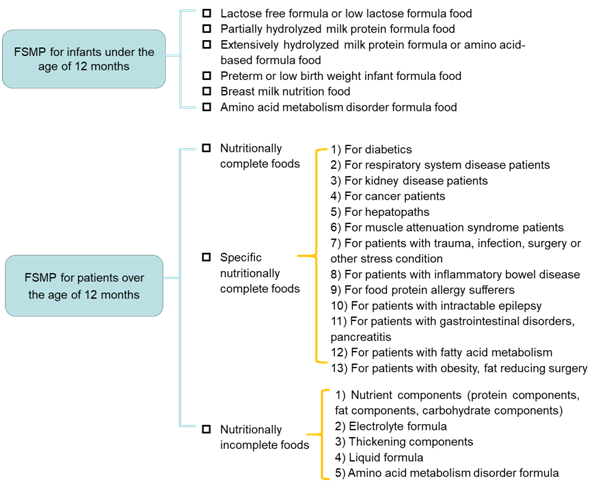
FSMP* | Food products that made from special processing formula and used to meet the special dietary need for people who have limited feeding, digestion absorption disorders, metabolism disorders or under specific disease condition. |
Drug | Products that used to prevent, treat and diagnose human diseases, purposefully regulate human physiological functions and prescribe indications, product usage and dosage. |
*The current FMSP categories in China are as follows:
2. Product usage
Category | Main usage |
General food | Provide energy and nutrients to human body, to maintain the normal metabolism. |
Health food |
|
FSMP |
|
Drug | Used to prevent or treat disease. |
3. Formula characteristics
Category | Formula characteristics |
General food | Contain proper nutrients, and are harmless to human body. |
Health food | I. Contain functional components, and will not cause any acute, subacute or chronic harm to human body. II. General food ingredients, approved new food raw materials, substances which are both used as food and drug in China, etc., are all allowed to use in health food. |
FSMP | I. Rich in nutrients (e.g., protein, fat, carbohydrate, vitamins, minerals, etc.), can meet the special dietary need for people under different disease condition. II. The product formula shall comply with GB25592/GB29922. Except for the nutrients and optional components stipulated in above standards, it is forbidden to add neither other bioactive substances nor drugs. |
Drug | Contain bioactive components, and are allowed to have side effects. |
Examples for the usage of food ingredients
I. Whey protein: It is a general food ingredient which can be used in general food, health food, and FSMP;
II. Cassiae semen: It is an ingredient which can be used as both food and drug, and it can be used in general food, health food, but not in FSMP.
4. Direction and dosage
Category | Direction | Dosage |
General food | Only by oral use | No specified limit |
Health food | Only by oral use | Have specific daily intake |
FSMP | Oral use or tube feeding | Shall be used under supervision of doctor or clinical dietitian |
Drug | Can be used by various ways, such as oral use, injection, applying, etc. | Has dosage limit, follow the doctor's advice |
5. Dosage form
Category | Dosage form |
General food | Have the corresponding forms that the food shall have, such as candy, chocolate, beverage, pastry, etc. |
Health food | Include tablet, capsules, oral liquid, granules etc., as well as some general food forms, such as beverage, pastry, candy, etc. |
FSMP | Similar to general food. At present, the most common dosage forms are powder and liquid. |
Drug | Tablet, capsule, granules, injections, topical coatings, topical ointments, etc. |
6. Product registration/filing
Requirements | Regulations | |
General food | No product registration or filing requirements before marketing | / |
Health food | Product registration or filing | Administrative Measures on Health Food Registration and Filing |
FSMP | Product registration | |
Drug | Product registration | Administrative Measures on Drug Registration |
7. Label claim
Category | Label claim |
General food | I. When the nutrient content reaches the requirements of GB 28050, it is allowed to make content claim or function claim of that nutrient on the label, and the expression shall be indicated in accordance with GB 28050. II. It is forbidden to claim health function or disease prevention/treatment. |
Health food | I. Indicate the approved health function on the label, such as “assisting blood sugar reduction”, “supplying vitamin C”, etc. II. It is forbidden to claim disease prevention or treatment. |
FSMP | I. Indicate the formula characteristic, and specify the suitable crowds in accordance with the product category. II. It is forbidden to claim health function or disease prevention/treatment. |
Drug | Claim the indications of the drug. |
8. Summary
From above 7 aspects, it is shown that there are essential differences between the food and drugs in China. For example, drugs are used to prevent or treat diseases, but food is forbidden to claim any disease prevention or treatment function; on the other hand, drugs are allowed to have side effect on human body, but food shall have no acute, sub-acute or chronic health effect to human body.
The main differences among general food, health food and FSMP are summarized in the following table. Food enterprises shall understand the definitions and purposes of these food categories before carrying out product R&D work and label preparation, etc.
General food | Health food | FSMP | |
Is health function allowed to claim? | No | Yes | No |
Can they prevent or treat disease? | No | No | No |
Are they designed to provide energy and nutrition support? | Yes | No | Yes |
Beside oral use, are other directions allowed? | No | No | Yes, only by tube use |
Do they have specific suitable crowds and daily intake limit? | No | Yes | Yes |
Shall they be used under supervision of doctor or clinical dietitian? | No | No | Yes |
Can substances which are both food and pharmaceutical in China be used in the products? | Yes | Yes | No |
Is it allowed to have side effects on human body? | No | No | No |

