Background
Food additive as an essential material in food industry, the quality and safety are always of concern. In accordance with Food Safety Law of the People's Republic of China, companies who plan to use new food additives (including enlarge the usage scope of existing food additives) or import new food additives to Chinese market shall submit the related safety assessment material to National Health Commission of the People's Republic of China (former NHFPC).
Which substance should apply for new food additive?
1) Food additives that have not been listed in GB 2760 Usage of Food Additives and GB 14880 Usage of Nutrition Enhancers;
2) Food additives that have not been listed in subsequent notices issued by NHC (former NHFPC);
3) Food additives of which the usage scope or dosage planned to be expanded.
Relevant laws and regulations

Registration dossier
1) Application form;
2) Common name, functional classification, dosage and application scope;
3) Materials or documents providing the technically necessity and using effect;
4) Qualification specification requirements and their test methods, manufacturing technique and test method of additive in food;
5) Safety assessment materials(when apply for expanding the usage scope or dosage, this material can be exempted);
6) Label and specification;
7) Other materials that contribute to the safety review, such as legal permission of producing and using from other countries (regions) or international organizations;
8) Power of attorney (when the registration application run by entrusted agencies);
As the first imported new food additive, besides the above documents, the following supplementary documents also should be submitted:
9) Certifying documents issued by government authorities or industry associations in the exporting country (region) of origin, proving that the new food additive is allowed to manufacture or sell in the country (region).
10) Certifying documents issued by government authorities or industry associations in the producing country (region) of origin, proving that the new food additive manufacturer has been examined or certificated.
Test requirements
Items | Illustration |
Quality specification test report | Three batches of new food additive shall be tested according to the quality specifications and inspection methods. |
Toxicology tests shall be proceeded according to GB 15193.1-2014. Or provide the toxicological evaluation material from overseas. |
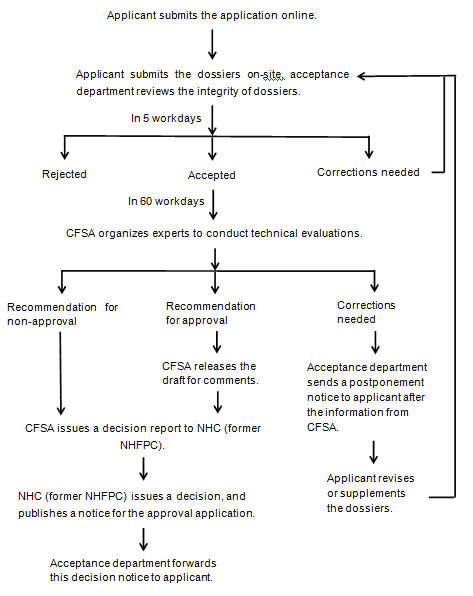
Work process of administrative approval
NHC: National Health Commission of the People's Republic of China
NHFPC: The National Health and Family Planning Commission
CFSA: China National Center for Food Safety Risk Assessment
Our services
1. New food additive registration
- Registration dossier preparation and submission;
- Foreign documents translation and notarization;
- Tests arrangements and monitoring;
- Project progress tracking;
- Attend the expert appraisal conference and assist enterprise answer the technical questions;
2. Training on new food additive registration
3. Other customized service
If you have any other questions, please feel free to contact us at service@cirs-group.com
