According to the interpretation of SAMR on 1 February 2021, the filing scope of health food made of coenzyme Q10, fish oil, melatonin, broken ganoderma lucidum spore powder, or spirulina as single active ingredient only includes the domestic products in China, and overseas products still need to apply for health food registration.
Therefore, in this article, CIRS compares the main differences between the registration and filing of imported health food to help relevant enterprises understand the advantages and disadvantages in the process of Chinese market access.
1. Scope of registration and filing for imported health food
Currently, the application scopes of registration and filing for imported health food are as follows:
- Scope of filing: the imported nutrition supplement of which the vitamins and (or) minerals meet the requirements in the Health Food Raw Materials Directory.
- Scope of registration: all imported health food (excluding nutrition supplement of which the vitamins and (or) minerals meet the requirements in the Health Food Raw Materials Directory)
2. Differences in required tests
The required tests for registration and filing are listed as follows:
Registration tests |
|
Filing tests |
|
Based on the above, compared with the domestic products that can apply for filing, imported products made of coenzyme Q10, fish oil, melatonin, broken ganoderma lucidum spore powder, or spirulina as single active ingredient shall carry out more tests to apply for registration, which mainly include:
- Coenzyme Q10 (improving immunity):toxicology test,animal function test;
- Coenzyme Q10 (anti-oxidation):toxicology test,animal function test, human feeding trials;
- Fish oil (assisting blood lipids reduction):toxicology test,animal function test, human feeding trials;
- Melatonin (sleep improvement):toxicology test,animal function test;
- Broken Ganoderma lucidum spore powder (improving immunity):toxicology test,animal function test;
- Spirulina (improving immunity):toxicology test,animal function test;
3. Differences in application dossiers
The biggest difference between registration and filing in the application dossiers is that, a comprehensive R&D report of the product shall be submitted for registration application. And as the requirements of filing are standardized from raw materials to products, the product R & D report is not required.
Therefore, imported health foods made of any of the five substances as single active ingredient such as Coenzyme Q10 shall provide the R&D report according to the registration requirements. However, as the 5 ingredients have a long history of consumption in China, and the product formula is well researched (the technical documents for domestic filing products can be taken as the important reference to prepare the R&D materials for the imported products), it will be much easier to compose the R&D report for them than for other functional health food.
Item | Registration | Filing |
Differences in dossiers | A comprehensive R&D report is required, which include, 1) Verification report of product safety; 2) Verification report of health function; 3) R&D report of production process; 4) Research on product technical requirements; | R&D report is not required |
4. Differences in application procedure
Registration procedure of imported health food
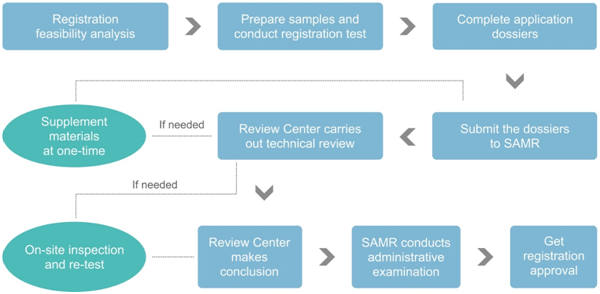
Filing procedure of imported health food
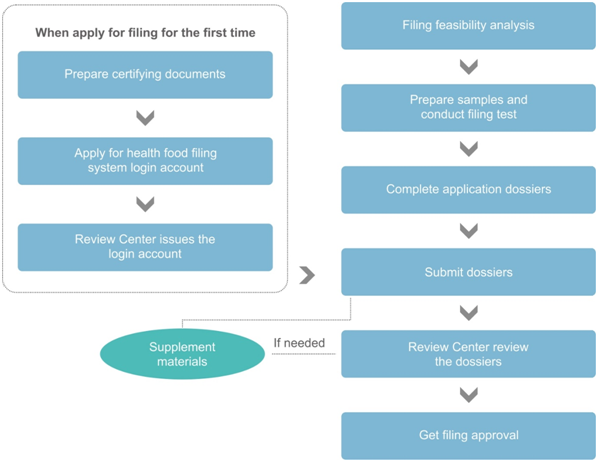
By comparing the above two flowcharts, the differences in the application procedures between the registration and filing mainly include the following four aspects:
Differences | Registration | Filing |
Application of the registration/fil-ing system login account | After filling in the information as required, the login account will be issued immediately, without manual review. | The overseas applicant shall prepare 3 certifying documents to apply for the login account. After passing the manual review of Review Center, the applicant can obtain the login account. |
Technical review | Required, and the technical review experts include,
| Not required, the filing dossiers will be reviewed by the officials from Review Center |
On-site inspection and re-test | Case by case, depends on the technical review results | Not required |
Administrative examination of SAMR | Required | Not required |
5. Comparison of estimated time
The estimated time for imported health food registration and filing are listed as follows:
Main procedures | Estimated Time (registration) | Estimated Time (filing) |
Feasibility analysis | 7 workdays | 3 workdays |
Prepare test samples | Depends on the applicant | Depends on the applicant |
Conduct registration/filing tests | 8-16 months (depends on the product function) | 4 months |
Complete application dossiers | 2 months | Within 1 month |
Acceptance agency accepts the application dossiers | Within 5 workdays | / (submit online) |
Review Center carries out technical review/filing review | Within 60 workdays | Generally 1-2 months |
Applicant supplements materials (if needed) | Within 3 months | Case by case (generally within 1 month) |
Review center carries out technical review/filing review once again | Within 60 workdays | Generally 1-2 months |
On-site inspection and re-test (if needed) | Case by case for imported products | / |
Review Center reports the review result to SAMR | Within 5 workdays | / |
SAMR conducts administrative examination | Within 20 workdays | / |
SAMR transfers materials to the acceptance agency | Within 3 workdays | / |
Acceptance agency sends registration certificate to the applicant | Within 10 workdays | Filing certificate will be issued by the filing system directly |
Total | 15+months | 8-10 months |
The overall duration of registration is longer than that of filing. However, due to the simple formula and sufficient demonstration of product safety, the registration of imported health foods made of any of the 5 substances as single active ingredient such as Coenzyme Q10 will be much smoother than other products demanding registration. It is expected that the registration certificates of these 5 products could be obtained in 15 months in the fastest.
On the other hand, the following two aspects will have impacts on the filing duration. Firstly, when applying for filing for the first time, the overseas applicant shall apply for the login account of filing system. Generally, after submitting the application, the Review Center will give feedback in 1 month. In fact, it is not easy to get a login account of filing system. If the applicant does not understand the requirements, it may take more than 1 year to obtain an account. Therefore, the account application should start as soon as possible. Secondly, after submitting the filing application, the dossiers will be reviewed by the officials from Review Center. Currently, there is no time limit specified for this step, and the review time is generally 1-2 months.
6. Conclusion
In summary, there are different provisions on registration and filing of imported health food in "application scope", "test requirements", "dossier requirements" and "application procedures", which lead to differences in the application duration and the cost. It is certain that the registration duration of health food is longer and the corresponding cost is higher.
However, the registration of imported health foods made of any of the 5 substances as single active ingredient such as Coenzyme Q10 is an exception. As we mentioned above, the preparation of R&D report of these products is no longer difficult, and the application of registration system login account is much easier than filing. Therefore, please do not hesitate to register these products in China. If you have certain demand, it is advised to prepare in time to seize the market of imported products.
If you have any needs or questions, please contact us at service@cirs-group.com.
