With the development of the epidemic and the arrival of rework peaks, timely detection of changes in body temperature can help to detect infected persons in advance. Since the thermometer is one of the emergency supplies, CIRS Group summarizes the technical requirements for the registration and declaration process of thermometer products based on medical device regulations and thermometer registration requirements.
According to medical device regulations, thermometers belong to the Class II medical devices and can be divided into infrared thermometers, electronic thermometers, and glass thermometers according to their working principles.
Overview of thermometer product
1. Infrared thermometer
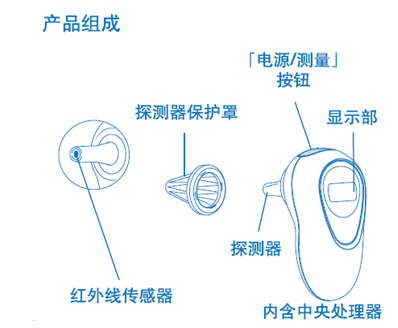
1.1 Structural composition of infrared thermometer:
It usually consists of infrared temperature sensor, probe cover, display unit, power supply circuit, and measurement circuit.
1.2 Infrared thermometer working principle:
In the natural world, all objects whose temperature is higher than absolute zero (-273 ° C) will emit infrared rays, and the energy of the infrared rays emitted is directly proportional to the temperature. Using this relationship, the temperature of an object can be calculated by measuring its infrared intensity.
1.3 Typical structure of infrared thermometer:
2. Electronic thermometer
2.1 Structure of electronic thermometer
It is generally composed of a detector (accessory of the probe protection cover), a sensor, a CPU control module, a display module, a tone module, and a power supply module.
2.2 Working principle of electronic thermometer:
The thermistor placed on the top of the measurement part is used as a temperature sensing device. When the temperature of the measured heat source changes, the resistance of the thermistor will change accordingly. The resistance of the thermistor in the measurement circuit by the internal microprocessor After the value changes, after conversion, processing and correction, the measured temperature will be displayed on the display screen in digital form, and at the same time, the beep will sound, and the measurement process will end.
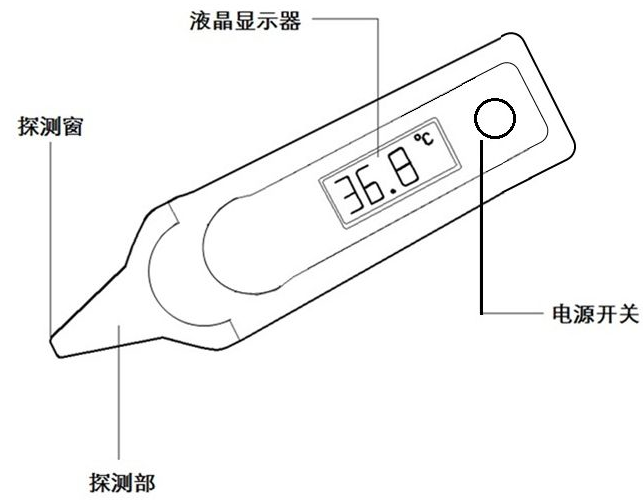
2.3 Typical structure of electronic thermometer products:
The product structure diagram is as follows
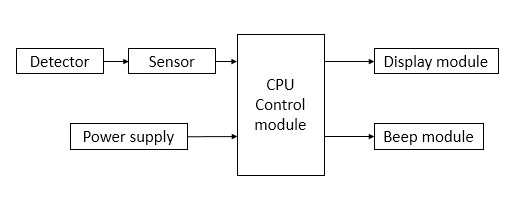
Figure 1 Product structure block diagram
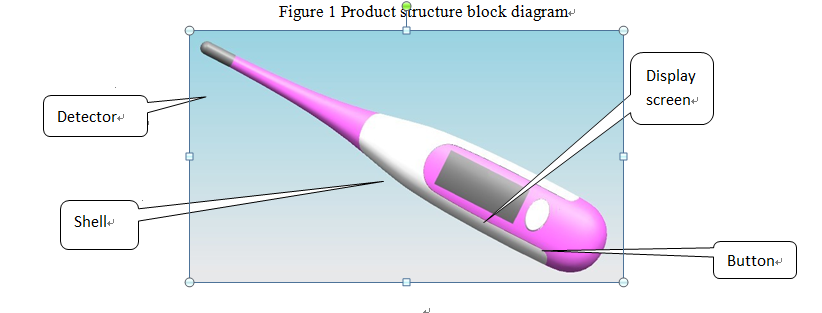
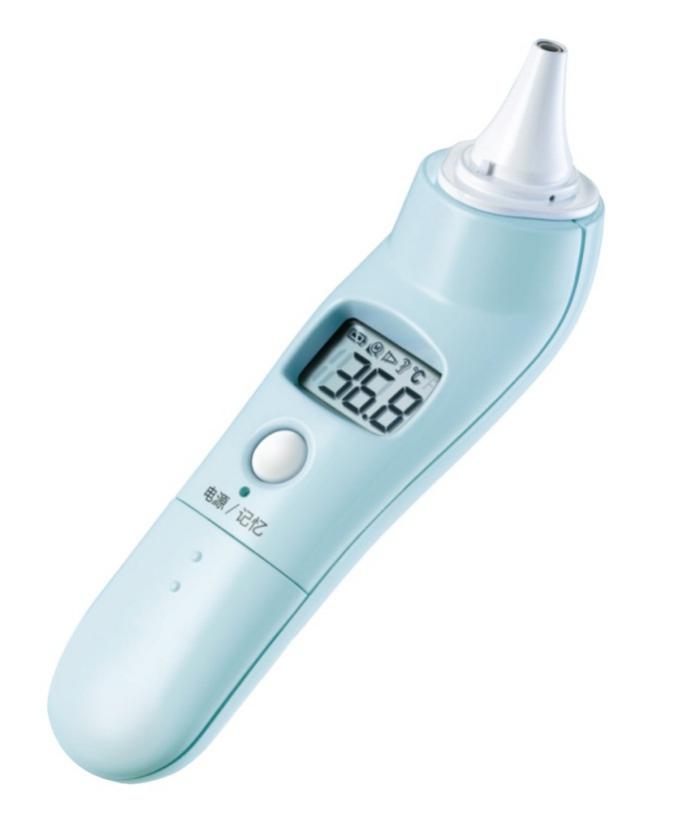
Figure 2 Product illustration example
3. Glass thermometer
3.1 Structural composition of glass thermometer
It is usually composed of glass tube, temperature sensing bubble, mercury or other temperature sensing liquid and scale.
3.2 Working principle of glass thermometer
The temperature is measured using the principle of thermal expansion and contraction of mercury or other liquids.
3.3 Typical structure of glass thermometer products:
Classification information Thermometer Product
Sub-catalog | First-level subdirectory | Second-level subdirectory | Product Description | Intended use | Product name | Management classification |
07 Medical examination and monitoring equipment | 03 Physiological parameter analysis and measurement equipment | 04 Body temperature measuring equipment | It is usually composed of glass tube, temperature sensing bubble, mercury or other temperature sensing liquid and scale. The temperature is measured using the principle of thermal expansion and contraction of mercury or other liquids. | For clinical measurement of patient temperature. It is usually placed on people’s mouth, underarms, and anus. | Glass thermometer, thermometer | Ⅱ |
It is usually composed of thermocouple or other contact temperature sensor, display unit, power supply circuit and measurement circuit. The sensor converts the temperature measured by contact conduction into an electrical signal for display or data output. | For clinical measurement of patient temperature. It is usually placed on the oral cavity, underarms, anus, and forehead of the human body. | Electronic thermometer | Ⅱ | |||
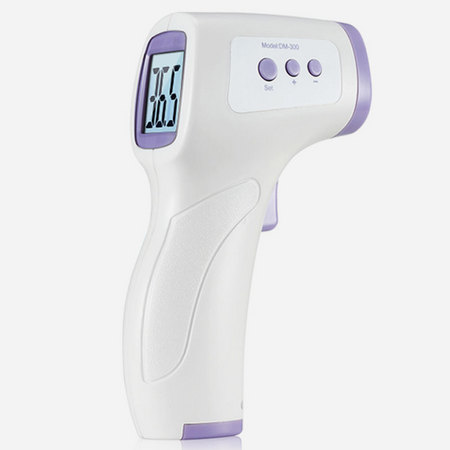
It usually consists of infrared temperature sensor, probe cover, display unit, power supply circuit, and measurement circuit. Use infrared temperature sensing method to measure temperature display or data output. | Clinical measurement of patient temperature with infrared method, usually used to measure the temperature of the patient's ear canal and forehead。 | Electronic thermometer, forehead thermometer, infrared ear thermometer | Ⅱ |
Clinical trial requirements for thermometers
The thermometer products have been included in the Medical Device Exempted from Clinical Trials Catalog, so they can be exempted from clinical trials, and only the clinical evaluation report needs to be submitted during the registration application process.
The thermometer products that are exempt from clinical trials are described as follows:
No. | Classification code | Product name | Product description | Classification |
353 | 07-03-04 | Forehead thermometer | It usually consists of infrared temperature sensor, probe cover, display unit, power supply circuit, and measurement circuit. Use infrared temperature sensing method to measure temperature display or data output. Clinically validated data should be provided for clinical accuracy. | Ⅱ |
354 | 07-03-04 | Body temperature measuring device (passive device) | It is usually composed of glass tube, temperature sensing bubble, mercury or other temperature sensing liquid and scale. The temperature is measured using the principle of thermal expansion and contraction of mercury or other liquids. For clinical measurement of patient temperature. It is usually placed on the body's mouth, underarms, and anus. Exemptions do not include products that use new materials, new mechanisms of action, or new features. | Ⅱ |
355 | 07-03-04 | Medical electronic thermometer | Medical electronic thermometer is an electronic device that monitors the patient's temperature intermittently. It can be composed of a plastic case, a circuit board, a temperature measuring part, a display screen, a power supply, etc.; it can be divided into several according to the design, technical parameters, additional auxiliary functions, intended use, etc. Model for measuring human body temperature or women's monitoring of ovulation cycle. It does not include prediction mode, nor does it include medical infrared thermometers. Product performance indicators use the applicable part of the following reference standards, such as: GB / T 21416-2008 medical electronic thermometer. | Ⅱ |
356 | 07-03-04 | Ear cavity medical infrared thermometer | The ear cavity medical infrared thermometer can be composed of a plastic case, a circuit board, a temperature measurement part, a display screen, a power supply, an isolation film, etc.; it can be divided into several models according to design, technical parameters, additional auxiliary functions, intended use, etc.; through heat radiation Display the temperature of the ear cavity of the measured human body. Clinical accuracy and clinical repeatability reports are required. Product performance indicators use the applicable parts of the following reference standards, such as: GB / T 21417.1-2008 medical infrared thermometers Part 1: Ear cavity type. | Ⅱ |
Registration approval cycle of thermometer products
In the case of general approval for thermometer products, the registration approval cycle is 80 working days (excluding the replenishment process):
Technical review: 60 working days, 60 working days after the replenishment;
Administrative approval: 20 working days.
CIRS Note: If registration is applied through the emergency approval process (green channel), the time for technical review and administrative review is expedited in accordance with provincial regulations, and the fastest review cycle may be accelerated to several working days to complete the review. However, the validity period of emergency approval documents is also stipulated, and the general period will not exceed one year.

