Recently, ECHA delivers the firsts results of enhanced completeness check. The original text is as follows.
Helsinki, 15 February 2017 – For 20 % (329) of the verified dossiers, companies were asked to improve the submitted information. More information was asked mainly on the following (one dossier may need to be improved in many areas):
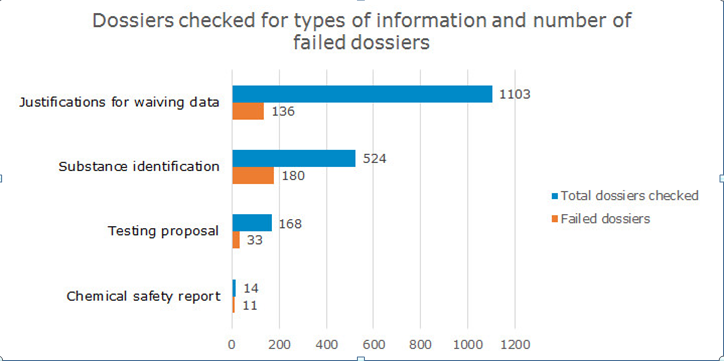
· Justifications for waiving data – 136
dossiers
Substance identification – 180 dossiers
Testing proposals – 33 dossiers
Chemical safety report – 11 dossiers
Almost all registrants (95 % of dossiers) updated their information as requested and passed the completeness check at their second attempt. The companies responsible for the remaining 5 % need to resubmit their dossiers with the missing information within the deadline set by ECHA. Failure to submit a complete dossier by the given deadline will mean that the submission is rejected, and, for first time submissions of registration dossiers, that a registration number will not be issued.
These statistics describe the situation from 21 June 2016 to 13 February 2017, and include both the first time submissions and updates to existing registrations.
To pass ECHA’s completeness check smoothly, companies are encouraged to use the IUCLID Validation assistant on their dossiers before they submit them to ECHA. The Validation assistant replicates the automatic part of the completeness check.
However, as the manual verifications are performed by ECHA staff, they cannot be replicated with the Validation assistant. Instead, more information on them including tips for companies is available on ECHA’s website. This information document on the manual checks was updated on 3 February with advice collected during the first six months of the enhanced completeness check’s operation.
Background
The enhanced completeness check has been in place since 21 June 2016. It is adapted to the improved data formats introduced by the launch of IUCLID 6. In the enhanced completeness check, the automated check is complemented by a verification by ECHA of those elements that cannot be checked automatically. This verification aims to make sure that registrants have justified any data waivers or deviations from the information requirements. In addition, ECHA staff will check that in their proposals to test on animals, registrants have included considerations on why none of the alternative methods could be used. Other areas verified are the substance identity and the presence of a chemical safety report when required. The enhanced completeness check applies equally to new registrations and updates of registrations.
Source: https://echa.europa.eu/-/enhanced-completeness-check-delivers-its-first-results

