With the release of Cosmetics Supervision and Administration Regulations on June 29, 2020, the National Medical Products Administration released the "Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments)" on July 21 for public opinions. Public feedback can be provided through the following channels and means:
Log in the Legal Information Website of the Ministry of Justice, PRC and go to the column "Solicitation of Legislative Opinions" on the main menu of the home page to submit the comments.
Mail to the following address: No. 1, North Luyuan Road, Xicheng District, Beijing, Postal code: 100037, and marked on the envelope "Comments on the Measures for the Administration of Cosmetics Registration and Filing".
E-mail: huazhuangpinchu@163.com.
The deadline for feedback is August 20, 2020.
The Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) have 88 articles in 7 chapters. It is divided into general provisions, basic requirements, cosmetics new raw materials registration and filing management, cosmetics registration and filing management, supervision and management, legal responsibility, supplementary provisions. CIRS sorted out and summarized the registration and filing management contents of new cosmetics raw materials in the Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) and the drafting instructions of Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments), and analyzed and interpreted the product registration experience over the years. The summary regarding the management of new cosmetic ingredient registration and filing can be found through the CIRS’s Interpretation of the Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) – New Cosmetic Raw Materials, which includes the main contents of Chapter 1 general rules and Chapter 2 basic requirements
The State implements registration management for special cosmetics and record management for ordinary cosmetics. The cosmetics registrant or the filing party shall have corresponding conditions. If the cosmetic registrant or filling party is an overseas enterprise, a legal person within the territory of China shall be designated as the domestic responsible person, and the domestic responsible person shall also perform relevant obligations. For details, please refer to the Article 10 and Article 11 of the Measures.
Chapter 4 of the "Measures" is the cosmetics registration and filing management (Article 36 to 64), which is divided into four parts: general requirements, filing management, registration management, and registration license renewal.
1. General requirements (Articles 36 to 44)
The content covers: raw material usage requirements, segmented production, imported cosmetic requirements, set products, entrusted confirmation, product implementation standards, registration filing inspection, efficacy data, merger and split of the company. Compared with the existing registration and filing requirements, revising the raw material usage requirements, and adding the product implementation standards and efficacy data. The guidelines on the basis of cosmetic efficacy claims shall be formulated and issued separately by the NMPA.
Table 1 Comparison of raw material usage requirements, product implementation standards and efficacy data in the "Measures" with relevant requirements of current laws and regulations
Item | Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) | Current regulatory requirements |
Raw material usage requirements | Clarify the source of raw materials and their raw material quality specifications. If the subsequent source of raw materials or raw material quality specifications change, safety assessment should be conducted and relevant registration and record information should be updated. | Raw material quality specifications are only available for high-risk raw materials. There is no need to update the registration and record information if the raw material quality specifications change. |
Product implementation standards | The cosmetics registrant and filling party shall formulate the standards for the implementation of the cosmetic products to be registered and filed. The ordinary cosmetics implementation standard shall be submitted to the NMPA for the filing of cosmetics, and the special cosmetic implementation standards shall be reviewed by the NMPA for the cosmetics registration. | Not required. But the documents such as product quality and safety control requirements are needed. |
Efficacy data | The efficacy claims of cosmetics should have sufficient scientific basis. Cosmetic registrants and filing parties can apply for registration or filing after obtaining the scientific basis of product efficacy claims. | The scientific basis of efficacy claims is only required for some special-used cosmetics such as SPF value. |
2. Filling management (Article 45-50)
2.1 New product filing process
The filing process of imported and domestic ordinary cosmetics is shown as below:
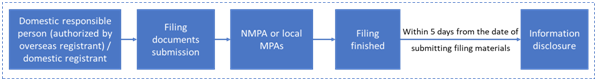
2.2 Change and cancellation of filing info
If the product name, formula, etc. of the filed products change, or the filing management department changes due to the change of the address of the filing party or domestic responsible person, the filing party shall cancel the original filing information for new filing application. If other items of the filed products change, it is required to apply for the change of filing. Moreover if the filed products are no longer produced or imported, the filer shall cancel the filing info.
1.3 Annual Report
After the filing of ordinary cosmetics, the filing party shall annually report the status of production, import and adverse reaction monitoring of the product to the NMPA.
When the management of imported non-special use cosmetics is changed from pre-market approval to pre-market filing, there is no valid date of the electronic certificate. And the domestic responsible person shall regularly report the production or import, marketing, monitoring of adverse reactions, and administrative penalties of the filed products to the NMPA through the online filing system platform every year. The "Measures" highlights the requirements of annual report for ordinary cosmetics after filing, and domestic ordinary cosmetics will also be included in the management.
3. Registration management (Articles 51 to 61)
3.1 New product registration process
Cosmetics used for hair dying, hair perming, anti-freckle and whitening, sun protection, anti-hair loss, and cosmetics that claim new effects are special cosmetics. Which are regulated under pre-market registration system. The soaps with the function of special cosmetics are subject to the management of special cosmetics.
The registration process of imported and domestic special cosmetics is as follows:

3.2 Compared with current regulatory requirements
The "Measures" that clarified the requirements for registration acceptance, registration withdrawal, and registration change are quite different from the current regulations.
Table 2 Comparison of the registration management of special cosmetics between the Measures and the current regulations
Item | Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) | Current regulatory requirements |
Authorized object of imported special cosmetics | Domestic responsible person | Chinese responsible person for notification |
Time limit of supplementary information | The applicant shall supplement the materials at one time within 90 days, and the technical review conclusion shall be made within 60 days from the date of receiving the supplementary materials. | The applicant submits supplementary materials in terms of the opinions of “Notice of Extension of Administrative License Technical Review" within one year, and the technical review period will be extended by 90 working days from the day of submission of all supplementary materials |
Time limit of re-review application | Applying for re-review within 20 days from the date of receipt of the notice. | Applying for re-review within 30 working days from the date of receipt of the "Notice of Administrative License Technical Review Opinion". |
Validity period of registration certificate | 5 years | 4 years |
Uploading sales package to the online platform | After the registration of special cosmetics or if the sales package is to be changed, the registrant shall upload the product sales package (including label and manual) pictures consistent with the label design sample through the online registration platform before the product is put on the market. After confirmation by the local MPAs, it will be disclosed to the public. | The sales package is no need to disclose to the public |
Change of registration info | Implement classified management on the degree of impact on product safety and efficacy.
| No classification management. Most of the changes need to be applied for the update. The original product name of imported cosmetics can’t be changed. New registration is required for the change of formula. There are no package changes in the registration system (except for changes in the SPF/PA index marked on the package). |
4. Renewal of registration certificate (Article 62 to 64)
If the special cosmetics registration certificate expires and needs to be renewed, the registrant shall submit an application for the renewal 30 days before the expiration of the registration certificate. If the renewal application is not submitted within the time limit, it will not be accepted. The general application process is as follows:
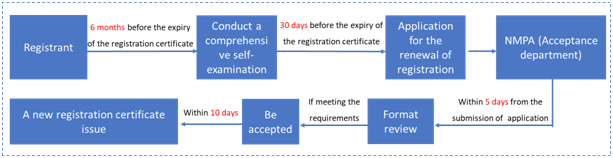
In May 2019, in order to further improve the official review and approval efficiency and consolidate the responsibility of the enterprises, the National Medical Products Administration issued the "Announcement on the Implementation of the Administrative License Renewal Commitment System for Special-use Cosmetics (Notice No. 45, 2019)". The announcement contained the requirements for the license renewal of the cosmetics administrative approval and gave examples of the self-examination commitment report for the license renewal of cosmetics.
In the current regulations, relevant regulations on the management of cosmetics registration and filing are scattered in multiple regulatory documents. Nevertheless, Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments), a supporting doc of the newly issued Cosmetics Supervision and Administration Regulations, includes the systematic and complete regulations on the cosmetics registration and filing. In addition to abovementioned contents of the Measures, the following contents show the innovative supervision systems and concepts of cosmetic products in other chapters of the Measures:
Handling of abnormal status of registration and filling (Article 69)
The relevant contact information provided in the online registration or filing system of the registrant, filing party, and domestic responsible person shall be updated in real time. In case the NMPA cannot contact the user through the filled contact info, it will be classified as an abnormal state.
If the cosmetics registrant, filing party, or domestic responsible person does not actively contact the NMPA within the specified time when the user account is restricted in an abnormal state, the drug supervision and administration department responsible for registration and filing management will cancel its registered and filing products.
Hierarchical Management (Article 71)
The drug supervision and administration department implements dynamic quantitative scoring based on the registrant, filer, domestic responsible person, the quality management system, the status of the recorded products, and the supervision and inspection situation of marked products so as to implement targeted supervision measures based on the scoring situation.
Domestic and imported cosmetics (Article 85)
The definition of imported and domestic products depends on whether the last process of contacting the contents is completed in China or overseas. If the products are filled in Taiwan, Hong Kong and Macau, they shall be managed as the imported products. For co-use or combination packaging products registered or filed under one product name, if the last production process of contacting the contents of any dose is completed overseas, it shall be managed as imported products. If the cosmetics are produced in stages at multiple addresses, the cosmetics registrant or filling party shall be responsible for the quality management of the entire process, and provide a complete production process when applying for the registration or filing certificate.
If you have any needs or questions, please contact us at service@cirs-group.com.
Source:
Measures for the Administration of Cosmetics Registration and Filing (in Chinese)

