With the release of Cosmetics Supervision and Administration Regulations on June 29, 2020. The National Medical Products Administration released the "Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments)" on July 21 for public opinions. Public feedback can be provided through the following channels and means:
Log in the Legal Information Website of the Ministry of Justice, PRCand go to the column "Solicitation of Legislative Opinions" on the main menu of the home page to submit the comments.
Mail to the following address: No. 1, North Luyuan Road, Xicheng District, Beijing, Postal code: 100037, and marked on the envelope "Comments on the Measures for the Administration of Cosmetics Registration and Filing".
E-mail: huazhuangpinchu@163.com.
The deadline for feedback is August 20, 2020.
CIRS sorted out and summarized the registration and filing management contents of new cosmetics raw materials in the Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) and the drafting instructions of Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments). The summary regarding the management of cosmetics registration and filing are summarized on the CIRS’s Interpretation of the Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) - Cosmetics.
The Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments) have 88 articles in 7 chapters. It is divided into general provisions, basic requirements, cosmetics new raw materials registration and filing management, cosmetics registration and filing management, supervision and management, legal responsibility, supplementary provisions.
Chapter 1: General Provisions (Article 1 to 7) |
Contents: basis of formulation, scope of application, definition, classification management, authority of national bureau, authority of provincial bureau, information disclosure |
Main Contents: 1) The definition of registration and filing
2)Define the product scope of classification management
3)Define the responsibilities of each department of the competent authority
|
Chapter 2: Basic Requirements (Article 8 to 13) |
Contents: the definition and responsibilities of registrants and filers, the requirements of cosmetic registrants and filers and the obligations of domestic responsible persons are clarified. An expert consultation mechanism has also been added. |
(1) Register and file new cosmetic raw materials in the name of the registrant and filer, and place the products on the domestic market; (2) Assist the registrant and filer in the monitoring of safety and reporting of new cosmetic raw materials; (3) Cooperate with the supervision and inspection work of the supervision departments.
|
Chapter III Registration and Filing Management of New Cosmetic Raw Materials includes the requirements of registration and filing management, safety inspection and reporting. The review procedures and timeline of pre-market registration and filing are stipulated. And the methods and requirements of post-market supervision are clarified and refined. CIRS Group has sorted out the application procedures for the registration and filing of different types of new cosmetics raw materials as follows:
Filing of imported new cosmetic ingredients
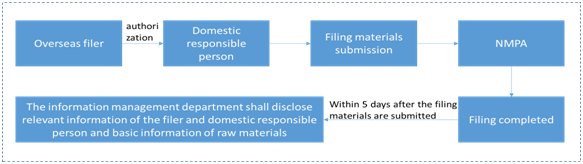
Filing of domestic new cosmetic ingredients

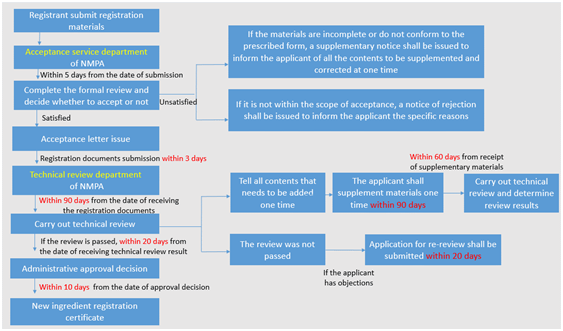
Registration of imported new cosmetic ingredients
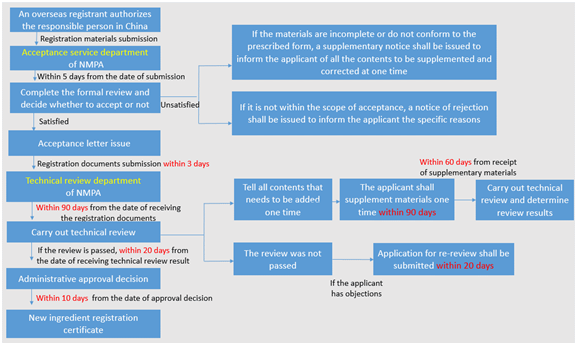
Registration of domestic new cosmetic ingredients
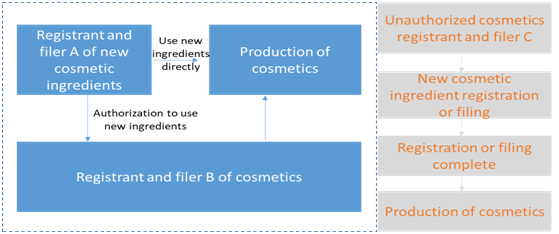
The State implements a safety monitoring system for new raw materials that have been registered and filed. The period of safety monitoring shall be 3 years since the date when the cosmetics using approved or filed new cosmetic raw materials for the first time have been registered or filed. Please refer to the following summary process on how to use and monitor the registered and filed new cosmetics raw materials:
The use method of new cosmetic ingredients that have been registered or filed with a safety monitoring period of less than 3 years
The use method of new cosmetic ingredients that have been registered or filed with a safety monitoring period of more than 3 years
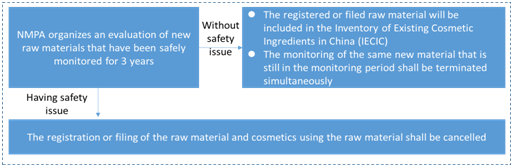
How to monitor the new cosmetic raw materials that have been registered or filed?
Both the registrants and filers of new raw materials and cosmetics produced with new raw materials shall perform corresponding monitoring responsibilities for the new cosmetic raw materials in the safety monitoring period. The specific monitoring work required by the registrants and filers of new raw materials and cosmetics produced with new raw materials as well as the monitoring work undertaken by the national and local Medical Products Administrations are summarized as follows:
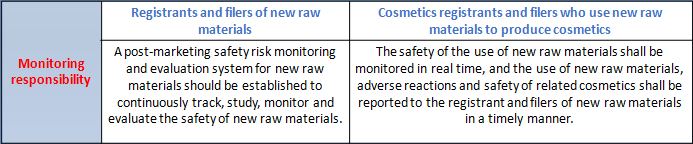
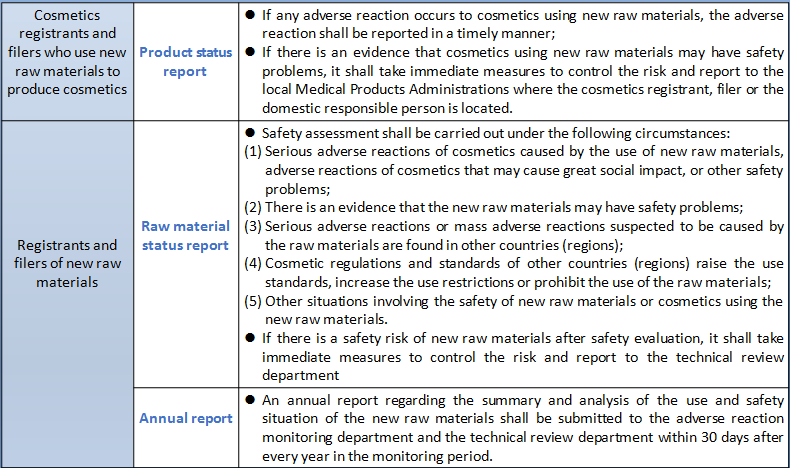
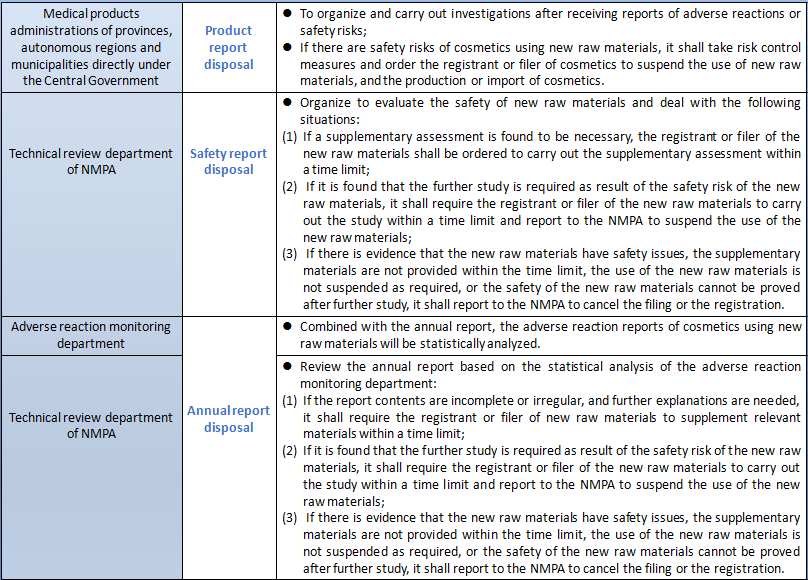
According to the drafting instructions of Measures for the Administration of Cosmetics Registration and Filing (Draft for Comments), the core of the Measures is to clarify the system framework, work responsibilities, basic systems, basic procedures and responsibilities of all parties for the registration and filing of cosmetics and new raw materials. It actively responds to the demands of the industry, clarifies the number and time limit of supplements, avoids prolonged review without decision, and specifies that filing will be completed upon the submission of filing materials to prevent disguised approval. The government will conscientiously check and fill in the gaps, clarify the responsibilities and obligations of registrants and filers as well as access conditions, and strengthen supervision of the source of product liability. The Measures conscientiously implement the requirements of the CPC Central Committee and the State Council for the reform of "delegation of power, control and service". New raw materials without high safety risk are put under the management of informed filing to accelerate the efficiency of the launch of innovative raw materials. It will focus on strengthening the provisions for safety monitoring period for new raw materials that have been registered or filed. The registrant and filer of new raw materials shall submit annual reports on the use and safety status of new raw materials to the adverse reaction monitoring department and the technical review department every year during the period of safety monitoring. In case of adverse reactions or possible safety issues of the cosmetics using the new raw materials, the cosmetics registrant and filer using the new raw materials shall submit a product status report, and the registrant and filer of the new raw materials shall conduct a safety assessment and submit a raw material status report. It is clear that the use of new raw materials in safety monitoring requires the consent of the registrant and filer of the new raw materials to protect the enthusiasm of new raw materials R&D enterprises. In the Measures, if the raw materials to be used in the production of cosmetics exceed the use purpose of use and the limit level of the used raw materials, it shall apply for the registration or filing of new raw materials. If safety differences are not involved, the safety monitoring period of the new raw material will not be set. That means the Inventory of Existing Cosmetic Ingredients in China may be managed to increase the use purpose and safe usage.
If you have any needs or questions, please contact us at service@cirs-group.com.
Source:
http://fgk.chinalaw.gov.cn/government_public/content/2020-07/21/657_3252817.html
http://www.moj.gov.cn/news/content/2020-07/21/zlk_3252815.html

