On June 18, 2021, the National Medical Products Administration issued the Provisions on Supervision and Administration of Children's Cosmetics (Draft, short for PROVISIONS) for public comments. On October 8, the "PROVISIONS" are officially released, and the regulations on children's cosmetics other than labeling requirements will come into effect from January 1, 2022.
Definition of children's cosmetics
Cosmetics with the function of cleaning, moisturizing, refreshing and sunscreen, etc. are applicable for children under 12 years old (including 12 years old).
Note: according to the Classification Rules and Catalogue of Cosmetics, the following effects are claimed for cosmetics suitable for children:
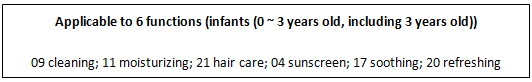

Principal responsibility
The registrant and filer of cosmetics shall be responsible for the quality, safety and efficacy claim of children's cosmetics.
Labelling requirements
- The display surface of the sales package shall be marked with the children's cosmetics logo specified by the NMPA (the children's cosmetics logo shall be published separately);
- Use "Caution" or "Warning" as a guide, and label warning words such as "should be used under adult supervision" on the visual surface of the sales package;
- Not allow to label the words such as "food grade" and "edible",etc or food related designs;
- Transition period of labeling requirements

Principle of formulation
- Safety first, efficacy essential, formulation minimalist
- Select cosmetic raw materials with a long history of safe use
- Ban to use new cosmetic ingredient under the monitoring period.
- Ban to use cosmetic ingredients prepared by new technologies such as gene technology and nanotechnology (Note: If necessary to use, please explain the reason and make safety evaluation of cosmetics used on children)
- Ban to use raw materials for the purposes of anti-freckle/whitening, anti-acne, hair removal, deodorization, anti-dandruff, hair loss prevention, hair dying and perming (Note: when using raw materials that may have the abovementioned effects for other purposes, the necessity of use and the safety of cosmetics used on children shall be evaluated)
- Focus on the scientificity and necessity of using raw materials such as flavours and fragrances, colorants, preservatives and surfactants.
Requirements of safety assessment
According to the "Technical Guidelines for the Safety Assessment of Cosmetic (2021 Edition), safety assessment and necessary toxicological tests are required.
Note: According to the "Regulations on the Administration of Cosmetics Registration and Filing Data", ordinary and special cosmetics used by infants and children are not exempt from animal testing.
Requirements of raw material inspection
The cosmetic registrant, filer and entrusted manufacturer shall strictly implement the a system of material purchase inspection record, carry out the testing of relevant items when necessary, and avoid bringing in prohibited raw materials such as hormones, anti-infective drugs or substances that may be harmful to human health through raw materials and packaging materials in direct contact with cosmetics.
Operating requirements
- Cosmetic operators shall establish and implement a system of purchase inspection record,
Checking:

Recording truthfully:

- Cosmetics operators should check the label information of the children's cosmetics they operate with the corresponding product information published on the official website of the National Medical Products Administration to ensure that the following information is consistent with the published information

Online business requirements
Cosmetics labelling information on the main page of business activities shall be consistent with the cosmetics registration or filing data;
The children's cosmetics logo shall be continuously publicized in a prominent position of the product display page.
Post market surveillance
Key regulatory objects: cosmetics registrants, filers, domestic responsible persons, entrusted production enterprises and cosmetics operators with concentrated sales of children's cosmetics
Key supervision contents: technical verification of product safety data;
Key supervision category: as the product category of annual sampling inspection and risk monitoring
Other requirements
- Avoid confusion between the properties, odour and appearance of children's cosmetics and food and pharmaceutical products to prevent accidental ingestion and misuse;
- Products marked with "applicable to the whole population", "used by the whole family" and other words, or the use of trademarks, patterns, harmonics, letters, Chinese Pinyin, numbers, symbols, packaging forms, etc. imply that the product is used by children in accordance with the management of children's cosmetics.
- Children's toothpastes shall be managed with reference to the “PROVISIONS”.
If you have any needs or questions, please contact us at service@cirs-group.com.

