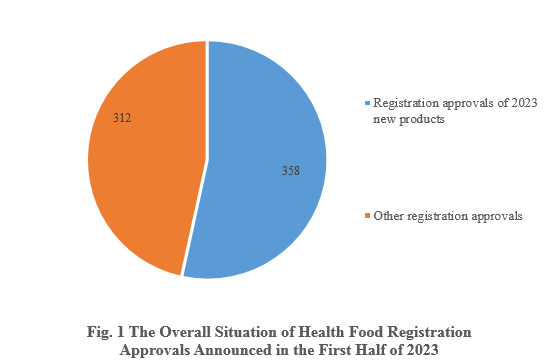
According to the information released by the Center for Food Evaluation, State Administration for Market Regulation and the Special Food Information Query Platform, in the first half of 2023, State Administration for Market Regulation issued a total of 670 health food (dietary supplement) registration approvals, 358 of which were for new health food products.
Related Links
(New) Analysis on Health Food (Dietary Supplement) Registration in 2023 in China
Statistics of health food filing in China in the first half of 2023
China Health Food Registration and Filing
We have summarized these 358 new products, and analyzed them from the following points:
- Overall situation;
- The number of health food registration approvals each month;
- The number of approved products in different regions;
- Enterprises obtaining health food registration certificates;
- The number of approved products with different dosage forms;
- The number of approved products with different health functions; and
- CIRS comments.
1. Overall Situation
Among the 670 registration approvals, 358 are new products, accounting for 53.43% of the total. Among the other 312 registration approvals, 17 of them are a transfer of technology registration, and the rest are possibly renewal of registration and change of registration.
2. The Number of Health Food Registration Approvals Each Month
As shown in Fig.2, in March, May, and June, there were 147, 101, and 74 new product registration approvals released respectively. And there are no new product approvals in the remaining months.
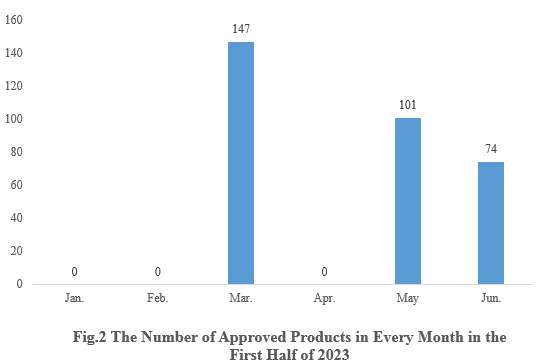
Note 1: There is a lag in the release of approval information, thus 36 products are not able to receive a specific approval date. These 36 products are not included in the statistics.
3. The Number of Approved Products in Different Regions
The 358 approved new products are from 28 provinces (municipalities and/or autonomous regions). Beijing, Guangdong, and Zhejiang occupy the top three spots with 76, 43, and 31 respectively, accounting for 21.23%, 12.01%, and 8.66% of the total new products.
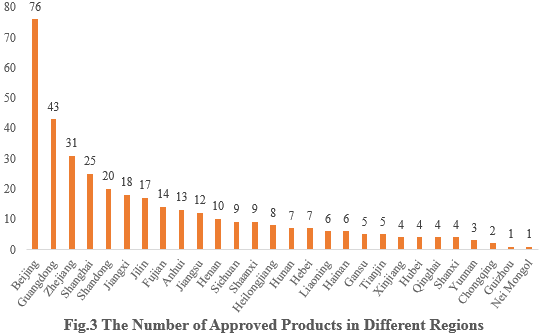
Note 2: If more than one enterprise from different provinces has applied for the registrations, all of them are included in the statistics.
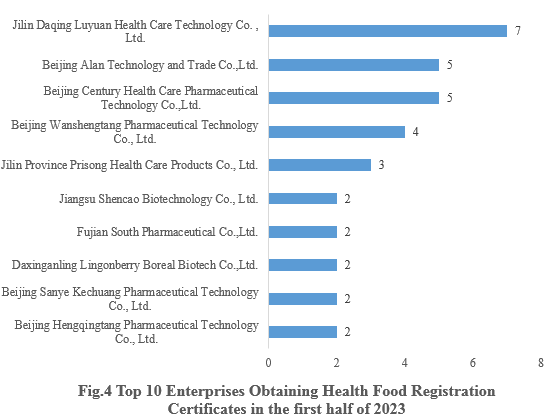
4. Enterprises Obtaining Health Food Registration Certificates
Jilin Daqing Luyuan Health Care Technology Co., Ltd., obtained seven health food registration certificates, ranking first in the first half of 2023. The top ten enterprises obtaining new registration certificates in the first half of 2023 are shown in Fig.4.
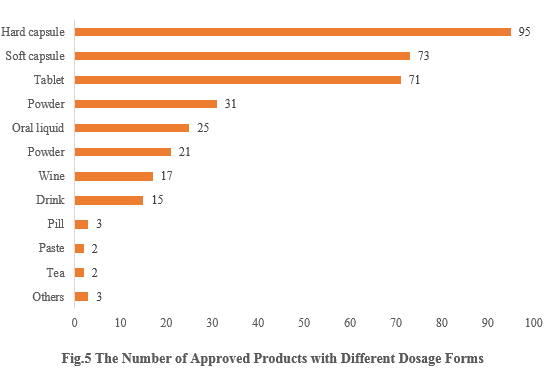
5. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved new products include capsules, tablets, powders, and oral liquids. Among them, capsule products received the largest number of approvals, with a total of 168 (including 95 for hard capsules and 73 for soft capsules), accounting for 46.39%. The number of tablet products is 71, accounting for 19.83%.
6. The Number of Approved Products with Different Health Functions
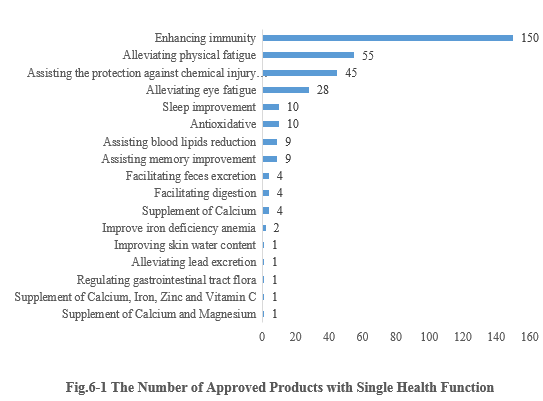
6.1 Single health function
As shown in Fig.6-1, enhancing immunity is the function with the largest number of approvals. The number of new products with the health function of enhancing immunity is 150, accounting for 41.90% of the total new products. Followed by 55 approved products with the health function of alleviating physical fatigue. It is worth noticing that six nutrient supplement products obtained registration approvals in the first half of this year.
6.2 Two health functions
Except for those new products with a single health function, there are also 20 products approved with two health functions (shown in Fig.6-2). It can be seen that most enterprises prefer to choose the function combination of “Enhancing immunity + X” for their products (“X” means other health functions besides enhancing immunity). And nine products with the function combination of “Enhancing immunity & Alleviating physical fatigue” received registration certificates in the first half of 2023.
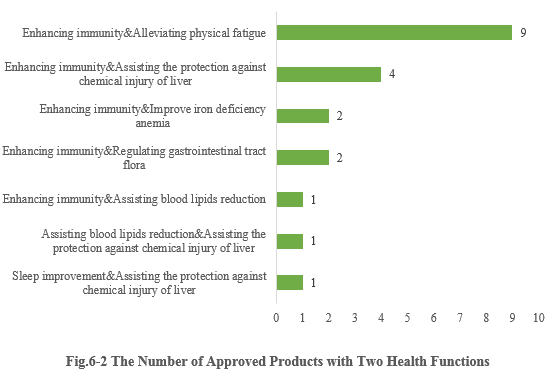
Note 3: Due to the lack of information in the Special Food Information Query Platform, the specific health function of three products cannot be found, thus these three products are not included in the above statistics.
7. CIRS Comments
Compared with the same period last year, the number of health food new products approved in the half of 2023 increased by 167%, which indicates the review of registered health food products is accelerating, and health food registration is gradually warming up. From the perspective of the enterprises, we suggest that enterprises focus on the nine currently available health function evaluation methods before the official release of the new evaluation methods, and make product applications according to consumers’ needs.
We have launched a one-stop platform for food ingredients and regulation data – ChinaFoodDB, which aims to provide pre-compliance services for users, including food ingredient inquiries, formula compliance self-checks, regulations, and announcements acquisition. It is a powerful tool for enterprises in the process of formula R&D and product innovation.
News Updates
In August 2023, China finally released the Directory of Health Functions Available to be Claimed by Health Food – Non-nutrition Supplements (2023 Version) and the supporting documents, including the long-awaited health food function evaluation methods, which includes a total of 24 available health functions. Compared with the earlier draft for public comment, the health claims allowed for functional health food have been reduced from the original 27 to 24. Three outdated functions, namely boosting lactation, promoting growth and development, and improving skin oiliness were formally removed.
Moreover, in accordance with the announcement of the State Administration for Market Regulation (SAMR) regarding the release of Directory of Health Functions Available to Be Claimed by Health Food - Non-nutrition Supplements (2023 Version) and the supporting documents, registrants need to change their health food registration certificates to revise the wording of their health claims, so as to align the latest wording and evaluation requirements when the health functions are listed in the Directory yet the wording of the health claims is outdated (e.g., regulating immunity or improving sleep).
If you need any assistance or have any questions, please contact us via service@cirs-group.com.
Data Source:
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.
Note:
There may be a lag in the data released in the Special Food Information Query Platform. The data in this article is only for reference, and the actual situation is subject to the official announcement.

