Since the nutrition supplement (health food) filing policy was implemented in 2017, CIRS has been summarizing the approved filing products every year. According to the data published by China authorities, the number of approved domestic nutrition supplements is much more than the approved imported nutrition supplements (see figure 1).
Based on the data, many overseas companies may wander why there is a big difference between the approval numbers of domestic and imported. Does that mean it is more difficult for imported nutrition supplement to get the approval? CIRS will compare the difference between the filing process for domestic products and imported products and emphasize the points for the attention for imported nutrition supplements.
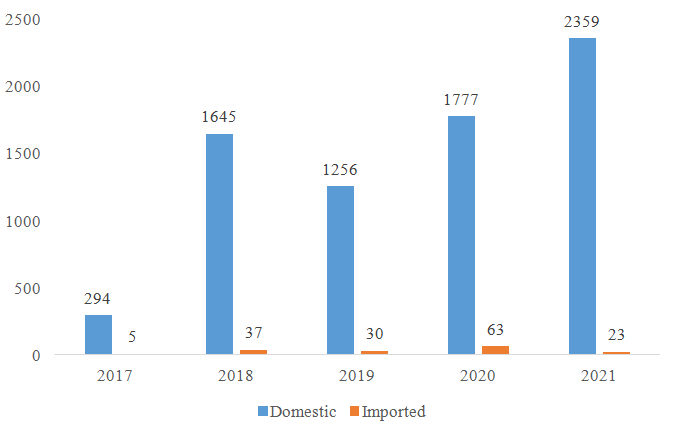
Figure 1: The number of filing approvals for domestic and imported nutrition supplement
Basic difference between domestic and imported filing process
Process | Domestic filing process | Imported filing process |
Responsible authority | Local Administration for Market Regulation | SAMR (national authority) |
Qualification of the applicant | Manufacturer | Overseas food companies (not necessarily to be the manufacturer) |
Scope | The ingredients list the filing directory, including 1. Vitamins and minerals; 2. Coenzyme Q10, broken Ganoderma lucidum spore powder, spirulina, fish oil and melatonin | Vitamins and minerals (listed in the filing directory) |
First of all, the responsible authorities are not the same. For domestic filing products, the approval is decided by the Local Administration for Market Regulations and most of them publish the approval information in time. However, for imported filing products, SAMR is the main platform for deciding their approvals and publishing the information, its information on the website is not that in time, which means that there are products that have already been approved, but the information has not been published right after. Besides, SAMR needs to review the products from all over the world, the review time takes longer than the domestic products.
Secondly, the qualification of the applicant is not the same. For China domestic filing process, the applicant has to be the manufacturer. In other words, who produces the products who could be the applicant. However, for the imported filing process, there is no limitation. The applicant could be the manufacturer but could also be the other companies such as product owner (entrust party).
Thirdly, for domestic products, they have more choices on the allowed ingredients. SAMR has expended the filing directory several times to include more ingredients allowed for the filing, but due to the the article 76 in the current food safety law, imported filing products could only choose the vitamins and minerals in the filing directory, not allowed for other functional ingredients.
The following part will introduce the process for imported nutrition supplement products filing process.
Filing process account application
For the filing application process, no matter for domestic products or imported products, the applicant need to get a filing system account from SAMR, and all applications shall be submitted using this account via the system. To shorten the time for process, CIRS suggest that the application for the filing account and filing product can be conducted at the same time, it is the fastest way.
Different from the domestic products, for foreign companies, the following documents shall be provided:
1. Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin, proving that the oversea filing applicant is the owner of the nutrition supplement marketed;
2. Letter of Authorization on the contact of nutrition supplements filling account application;
3. A scanned copy of the passport of the legal representative of the applicant
When preparing those documents, there are many details that need to be paid attention to. For example, qualification certifying documents and letter of authorization have to be notarized and confirmed by China embassy.
Required documents for product filing
In general, the required documents for domestic products and imported products are quite similar, but compared with domestic products; there are more documents for imported products:
Additional documents for imported products:
1. Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the filing applicant is the oversea manufacturer of the health food marketed;
2. Certifying documents proving that the product has been marketed more than one year as food supplement;
3. Nutrition supplement-associated standards, self-inspection reports of the manufacturer and GMP certificate;
4. Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
5. Letter of authorization if the filing process is entrusted by agency.
For overseas companies, the preparation of those documents usually take longer than expected.
Who will issue the required documents? Any points have to be included in those documents? When preparing the requirements, there are many points need to emphasized and paid attention to. Otherwise it might lead to re-preparation, which takes longer.
Summary: difficulties for imported nutrition supplement product filing in China
Based on the filing data published, there is a huge difference in the number of approvals for domestic and imported products. The reasons are multiple, the number of domestic applications are much more than imported applications, and the review for domestic products and the information share is much quicker. Regarding the required documents, process, required tests, those are quite similar for domestic and imported products. However, for imported nutrition supplement companies, the application of nutrition supplement filing account and product filing may still face the following difficulties:
1. Deep understanding of Chinese regulations: In China, there are lot of rules and regulations related to nutrition supplement filing, and the content is exactly rich. Only regulatory specialists who are very familiar with the regulations can have a comprehensive understanding and make as few detours as possible.
2. Rich practical experience: Besides the regulation requirements, CIRS found that some specific details may not be explained in regulations, but are still required by the review experts of nutrition supplement filing. Therefore, regulatory specialists who are responsible for the filing affairs shall have the rich experience and communicate with review experts frequently, so as to get the detailed requirements.
3. Tracking regulations and policies: nutrition supplement regulation is updating all the time, and the review experts are becoming stricter on the submitted dossiers. In CIRS Food Division, we have dedicated personnel to monitor the updating regulations and notify everyone. Meanwhile, when dealing with a new project, if any regulatory specialist finds that the requirements have changed, the one shall notify the others to avoid detours in subsequent projects.
To overcome those difficulties, it will take overseas companies a lot of time and money. Therefore, almost all imported products will choose a trustful regulatory agency to assist with the process. CIRS is qualified and experienced in doing those regulatory affairs for overseas companies. If you have any questions or remarks, please feel free to contact us.
Free Webinar: Latest Updates of China Health Food (Dietary Supplement) Registration/Filing Regulation and Practical Skill Sharing

CIRS is to host the free webinar on dietary supplement from 22nd to 23rd of March, 2022. The webinar includes four topics, assisting the companies to keep up with the latest topics and regulation updates. The webinar will be presented in three languages (Chinese, English and Japanese). Look forward to your participation.
- Topic 1: Latest Updates of China Health Food (Dietary Supplement) Regulations;
- Topic 2: Imported Health food (dietary supplement) Filing Practical Skill Sharing
- Topic 3: Key Points and Challenges of Health Food (Dietary Supplement) Registration;
- Topic 4: Latest Requirements and Common Mistakes of Health Food (Dietary Supplement) Labels
Check here for more details about the webinar.
If you have any needs or questions, please contact us at service@cirs-group.com.

