
According to the requirements of laws and regulations in China, the health function claims for health food (dietary supplement) shall be listed in the health food function claim catalog. Up to now, a total of 24 health food function claims have been approved in China and listed in the health food function claim catalog. Enterprises need to select the function claims that match their products from the health food function claim catalog.
In order to further standardize the management of health food function claim catalog, China SAMR issued and implemented the Administrative Measures for the Catalogue of Health Food Raw Materials and Health Function Catalogues in 2019 (hereinafter referred to as the "Administrative Measures"), which stipulated that any organization or individual could make suggestions on the supplementation of health food raw materials and health food function claims on the basis of scientific research and demonstration.
On August 2 of 2022, SAMR issued the "Implementation Rules for New Functional Technology Evaluation of Health Foods (Trial) (Draft for Comment)" (hereinafter referred to as "Implementation Rules"). The "Implementation Rules" is a supporting document for the Administrative Measures. It further standardizes the requirements for the application of "new health function claims of health food". The draft is open for public comments until 1 September 2022.
CIRS Group makes a systematic introduction to the existing application requirements for "new health function claims of health food" as follows:
1. Related regulations
Regulation | Status | Date |
Administrative Measures for the Catalogue of Health Food Raw Materials and Health Function Catalogues | Effective | 2019.10.01(implement) |
Implementation Rules for New Functional Technology Evaluation of Health Foods (Trial) (Draft for Comment) | Draft | 2022.08.02 (publish) |
Note: The requirements on health food new function in this article refer the “Implementation Rules" (draft) and "Administrative Measures". The requirements in the final version might be different
2. Requirements for the health food new function claim application
I. Qualification requirements
- Applicants of new health function claims could be any organization or individual;
- Individuals shall cooperate with organizations that are qualified for health food registration to propose suggestions for new health food function claims.
II. Scope of new health function claims
New health function claims shall fall within one of the following categories:
(1) Providing the nutrients;
(2) Maintaining or improving the health conditions;
(3) Reduce the risk of certain disease.
III. Application flow chart
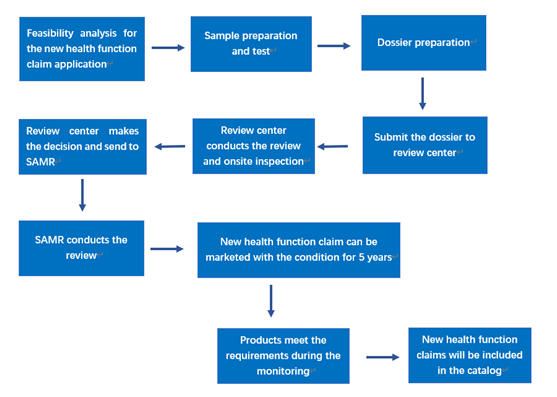
IV. Requirements for the application materials
Materials required for new health claim applications
- Table of contents of new health function claim;
- Letter of commitment for authenticity;
- Copy of the ID card or registration certificate of the applicant;
- Name, explanation, and supporting evidence of the proposed new health function claims;
- R&D reports of the new health function claim;
- Evaluation and verification materials of the new health function claim;
- Same or similar health function in the application in China and other countries;
- Other scientific supporting evidence;
- Materials related to the ethics of health function claim;
- Technical evaluation for the samples;
- Other materials related to the function evaluation;
- Other materials related to clinical studies;
- Other issues need to be mentioned;
V. Test items required
The test items required for the new health food function claim application listed as follows:
Test items for the new health food function | |
Test items | Note |
Verification of the health function claim evaluation method | Before proposing the new health function claim, the products shall at least pass the evaluation of one lab 1) In principle, the evaluation shall include human trial test; 2) For function claims has been approved in more than 3 counties, the applicant shall provide the function evaluation method which is widely recognized internationally, and provide the test reports of at least 1 lab. 3) For function claims has been approved in only a few countries, or by a few of companies, applicant requires to carry out the evaluation study in multiple labs, and provide the test report of at least qualified 1 lab. |
During the monitoring period of the products with new health function claims, shall randomly pick labs to do the new health function claim evaluation, and before the monitoring period ends, shall provide the test report of at least qualified two labs. | |
Other required tests | |
Self-inspection test | |
Active ingredient test, hygiene test, and stability test | |
Active ingredient test and test method validation | |
Toxicology test | |
Animal function test and (or) human trail test | |
Other test (if necessary) | |
3. Summary
Currently, as there are only 24 health function claims approved in China, companies may find it difficult to choose suitable health functional claims for their products. However, since the Implementation Rules for New Functional Technology Evaluation of Health Foods (Trial) (Draft for Comment) was published, the new health function claim application becomes possible. Although it is still a draft version, we believe that companies could apply for the new health function claims in the near future.
If you have any needs or questions, please contact us at service@cirs-group.com.

