With the growth and aging of China's population, the patients of clinical diseases, such as the diabetes, kidney disease, cancer, obesity, chronic obstructive pulmonary disease,and amino acid metabolic disorders , gradually increased and they have demands for special nutrients or dietary food. Foods for Special Medical Purpose (FSMP) are made from special processing formula and used to meet the special dietary need for people who have limited feeding, digestion absorption disorders, metabolic disorders or specific disease condition. FSMP is registered as drug in China now and the high registration fee causes high price to limit its development in China. To meet clinical demands of FSMP and reduce the economic burden for patients, the Chinese government is gradually set up related supporting system of the national standard in line with international standards,
CFDA released the Administrative Measures of FSMP Registration (trial) (draft) on September 2, 2015 (for public comments by on October 1, 2015).The regulation has specific provisions on the FSMP registration procedure and requirements. CIRS speculated that the final version of the Administrative Measures of FSMP Registration (trial) will have slight modification compared with this draft version. Now the CIRS provide the detailed explanation.
1. What is the use scope of FSMP?
CFDA released the Administrative Measures of FSMP Registration (trial) (draft) on September 2, 2015 (for public comments by on October 1, 2015).The regulation has specific provisions on the FSMP registration procedure and requirements. CIRS speculated that the final version of the Administrative Measures of FSMP Registration (trial) will have slight modification compared with this draft version. Now the CIRS provide the detailed explanation.
1. What is the use scope of FSMP?
FSMP must be used alone or with other food under the guidance of the doctors or clinical nutritionists. FSMP belongs food, not drugs, and cannot be a substitute for drugs. So this kind of products cannot be claimed for disease prevention and treatment function.
| Country or Organization | Chinese name | English name |
| The Codex Alimentarius Commission(CAC) | 特殊医学用途食品 | Foods for Special Medical Purpose |
| EU(European Union) | 特殊医学用途膳食食品 | Dietary foods for special medical purposes |
| America & Canada | 医用食品 | Medical Foods |
| Australia | 特殊医学用途食品 | Foods for Special Medical Purpose |
| Japan | 病人用特别用途食品 | 特別用途食品(Japanese name) |
| China | 特殊医学用途配方食品 | Foods for Special Medical Purpose |
2.What kinds of FSMP required to be registered?
| Age Groups | Categories | Concrete Implication |
| FSMP for the age 1 year older and above. | Full nutritional formula food | These products can be used as a single source of nutrition for targeted population requirements. |
| Specific Full nutritional formula food | These products can be used as a single source of nutrients to meet targeted groups requirements in a particular disease or medical condition. There are 13 categories of specific full nutritional formula foods below: 1. Full nutritional formula food for diabetics; 2. Full nutritional formula food for respiratory system disease patients, 3. Full nutritional formula food for kidney disease patients; 4. Full nutritional formula food for cancer patients; 5. Full nutritional formula food for hepatopaths, 6. Full nutritional formula food for muscle attenuation syndrome patients, 7. Full nutritional formula food for patients with trauma, infection, surgery or other stress condition, 8. Full nutritional formula food for patients with inflammatory bowel disease; 9. Full nutritional formula food for food protein allergy sufferers;10. Full nutritional formula food for patients with intractable epilepsy; 11. Full nutritional formula food for patients with gastrointestinal disorders, pancreatitis; 12. Full nutritional formula food for patients with fatty acid metabolism, 13. Full nutritional formula food for patients with obesity, fat reducing surgery. | |
| Part nutritional formula food | These products can be used to meet the targeted population requirements of part nutrition and cannot be a single source of nutrients. There are 5 categories of part nutritional formula food below: nutrient components (protein components, fat components, carbohydrate components), the electrolyte formula, thickening components, liquid formulation and amino acid metabolism disorder formula. | |
| FSMP for 0 to 12 months old infant. | No lactose formula or low lactose formula food | |
| Partially hydrolyzed milk protein formula food | ||
| Completely hydrolyzed milk protein formula food or amino acid formula food | ||
| Premature delivery or low birth weight infant formula food | ||
| Amino acid metabolism disorder formula food | ||
| Breast milk nutritional supplements | ||
3.What are related national standards about FSMP?
| S.N. | National standards |
| 1 | The General Rules of Foods for Special Medical Purpose (FSMP) (GB 29922-2013)and related Q&A. |
| 2 | Food Safety National Standards for the General Rules of Infant Formula Foods for Special Medical Purpose (FSMP) (GB 25596-2010) and related Q&A. |
| 3 | Good Manufacturing Practices of Foods for Special Medical Purpose (FSMP) (GB 29923-2013) |
| 4 | Food Safety National Standards for Labeling of the Prepackaged Foods for Special Dietary uses(GB13432-2013) |
| 5 | The Administrative Measures of Foods for Special Medical Purpose (FSMP) Registration (trial) |
| 6 | Clinical Trials Quality Control Specifications of Foods for Special Medical Purpose (FSMP) |
| 7 | The Administrative Measures of Food Production License |
| 8 | The Administrative Measures of Food Operation License |
| 9 | The Provisional Regulations on Food Advertising (revised) |
| 10 | Advertisement Law of People’s Republic of China (2015 version) |
| 11 | Food Safety National Standards for the Uses of Food Additives(GB 2760-2014) |
4. What requirements dose the applicant have to meet to apply for FSMP registration?
FSMP production and marketing manufacturers in China and foreign FSMP manufacturers that want to export to China can apply for FSMP registration. The applicants above must meet all the following three requirements:
(1) Set up the independent R&D institutions and should be equipped with full-time personnel and equipments;
(2) Must meet good manufacturing practices standards requirements (GB 29923-2013);
(3) Have the ability to complete all test items according to the quality standard.
5. Which kinds of FSMP need clinical trials?
All the specific full nutritional formula foods should be completed the clinical trials, but other categories of FSMP do not need to do clinical trials.
6. What are qualified clinical trial methods and the technical indicators based on?
The Clinical Trials Quality Control Specifications of Foods for Special Medical Purpose (FSMP) are the basis for qualified clinical trial methods and the technical indicators. These specifications will be released by CFDA.
7. How many clinical test institutions are there?
The clinical trial institutions will be certificated and released by CFDA. Applicants can select several clinical trial institutions (not more than five ) to do clinical test and the lead institutions and the statistical institutions should be clear.
8. How many sampling test institutions are there?
The list of FSMP sampling test institutions will be released by CFDA.
9. What content the on-the-spot inspections have?
CFDA will inspect R&D ability, production ability, test ability and so on by reviewing the materials, paying a return visit to the clinical trials crowds and other ways.
10. What content are listed in the eye-catching place of FSMP label and instruction?
All the following contents should be appeared:
(1) This product should be used under the guidance of doctors or clinical nutritionists;
(2) This product is only used for the targeted population;
(3) This products cannot be used for parenteral nutrition support and intravenous injection.
11. The procedures of FSMP registration.
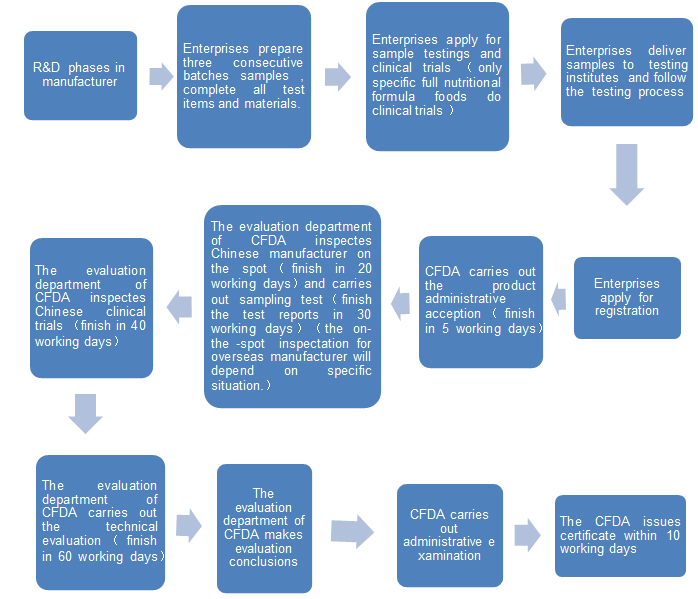
12. The required materials of FSMP registration.
| No. | Registration required materials |
| 1 | The application form of FSMP registration |
| 2 | Products R&D reports, formula materials and formula design basis |
| 3 | Product process materials |
| 4 | Product quality standards |
| 5 | Product labels and manual sample |
| 6 | Test reports |
| 7 | Evidence materials of R&D ability, production ability and test ability |
| 8 | Other evidence materials of product safety and nutritional adequacy |
| 9 | Clinical trial reports (only specific full nutritional formula foods do clinical trials ) |
| No. | CIRS predicts additional materials that required for imported FSMP registration |
| 1 | The certificates which demonstrate that the registrant is the legal possessor of the product. The certificates should be issued by the countries or regions where manufacturer belong to and have the valid period on it. |
| 2 | The certificates which certify that manufacturer meets the local quality management standards and the products have been marketed in the original country. These certificates should be issued by original country and have the valid period on it. |
| 3 | If foreign manufacturers entrusts Chinese company to registrant FSMP, the Chinese company should provide entrust documents, notarial documents and Chinese "business license" copies. |
| 4 | Product usage and adverse reaction summary. |
| 5 | The package, labels and manual samples of the product in original country or region. |
| 6 | The product-related standards from original country or international organizations. |
| 7 | Three conductive batches of on sale products or specially treated samples which used in review testing. The number is triple to the quantity needed by testing.。 |
| 8 | All foreign language materials provided by manufacturers need to be translated into Chinese. And Chinese document should be notarized by Chinese notary department. The certificates provided by foreign institutes should be notarized by local notary department and need to be confirmed by Chinese Embassy. The product quality standards (Chinese version) have to be in line with the format of Chinese quality standards of FSMP. |
This new regulation of FSMP registration simplifies the registration procedure and shortens the registration duration to provide more opportunities and convenience for manufacturers to enter into Chinese market.
If you have any questions or recommendations about the new FSMP registration regulation, please don’t hesitate to contact us. We are ready to offer help or convey your comments to CFDA.
Contact us:
Ms. Cathy Yu Team Leader of Food Safety and Regulatory Affairs Department, CIRS China
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Tel : +86 571 8720 6538 | Fax : +86 571 8720 6533
Email: cathy.yu@cirs-group.com






