When importing or manufacturing hazardous chemicals or mixtures containing them in Asia, it is not just a case of translating labels and safety data sheets (SDSs) into the local language, it is also important to consider local GHS standards.
In South Korea there are several authorities involved in implementing the globally harmonized system for classification and labelling of chemicals and mixtures (GHS):
- The Ministry of Education and Labor (MoEL) is responsible for the occupational safety and health act;
- Korean Occupational Safety and Health Agency (KOSHA) is responsible for reviewing/authorizing material safety data sheets (MSDSs); and
- The Occupational Safety and Health Research Institute (OSHRI) is responsible for research and development guidelines.
In November 2020, South Korea’s Ministry of Education and Labor (MoEL) made updates to its regulation, which aligns the country with the UN GHS. MoEL No 2020-130 went into force on January 16, 2021, ushering in the mandatory requirement for submitting an MSDS as well as the procedure for the protection of confidential business information (CBI).
All companies are required to submit an MSDS and failure to do so will result in a fine of up to five million South Korean Won (approximately 3,700 Euros) per product.
Certain products are exempt, including those:
- controlled under other laws;
- consumer products; and
- chemicals or products for research and development (R&D) purposes.
Grace periods
The regulation came into effect on January 16, 2021, however not all companies were required to submit an MSDS from that date.
New chemicals – Companies importing or manufacturing hazardous chemicals into South Korea for the first time, need to submit a compliant MSDS before manufacture/import.
Existing chemicals – Enterprises that were importing or manufacturing hazardous chemicals into South Korea before the updated regulation came into force, must update and submit their MSDS before the applicable grace period.
The grace period depends on the annual tonnage of the substance:
- ≥ 1,000 tons – January 1, 2022.
- 100 – 1,000 tons – January 1, 2023.
- 10-100 tons – January 1, 2024.
- 1-10 tons – January 1, 2025.
- <1 ton – January 1, 2026.
If any information in the MSDS changes, then related enterprises must submit the updated MSDS to MoEL as soon as possible. South Korea has adopted a 16-section MSDS, which aligns with the fourth revision of GHS. The MSDS and label must be authored in Korean. Unlike in China, the emergency contact number does not need to be a 24hr local number, however, a local number is preferred.
MSDS submission code
The MSDS must include a submission code in the upper right-hand corner. To obtain this code, companies must first produce a qualified Korean MSDS and then submit it to authorities for review. After that, the system will generate a submission code, which can be added to the MSDS, and then the companies are compliant with the standards.
Confidential Business Information (CBI)
Companies are required to provide 100% composition information on the MSDS. However, this does not mean the specific concentration must be included, instead, a concentration range is permitted:
- If the concentration of a substance is below 25% the range is &pm10%; and
- If it is equal to or above 25% the range is &pm20%.
CBI protection is normally only valid for five years. So if CBI is granted, companies must include the authorization number and expiry date that was provided by the MoEL. If they wish to extend CBI protection they will need to re-apply at least a month prior to the expiry of the five-year period.
For the application of CBI protection, the following information is required:
- Applicant information;
- Reason for requesting CBI protection;
- Alternative data (generic name, concentration range);
- 100% ingredient information (substance name and concentration) and hazardous substance information;
- MSDS; and
- Other documents required by MoEL.
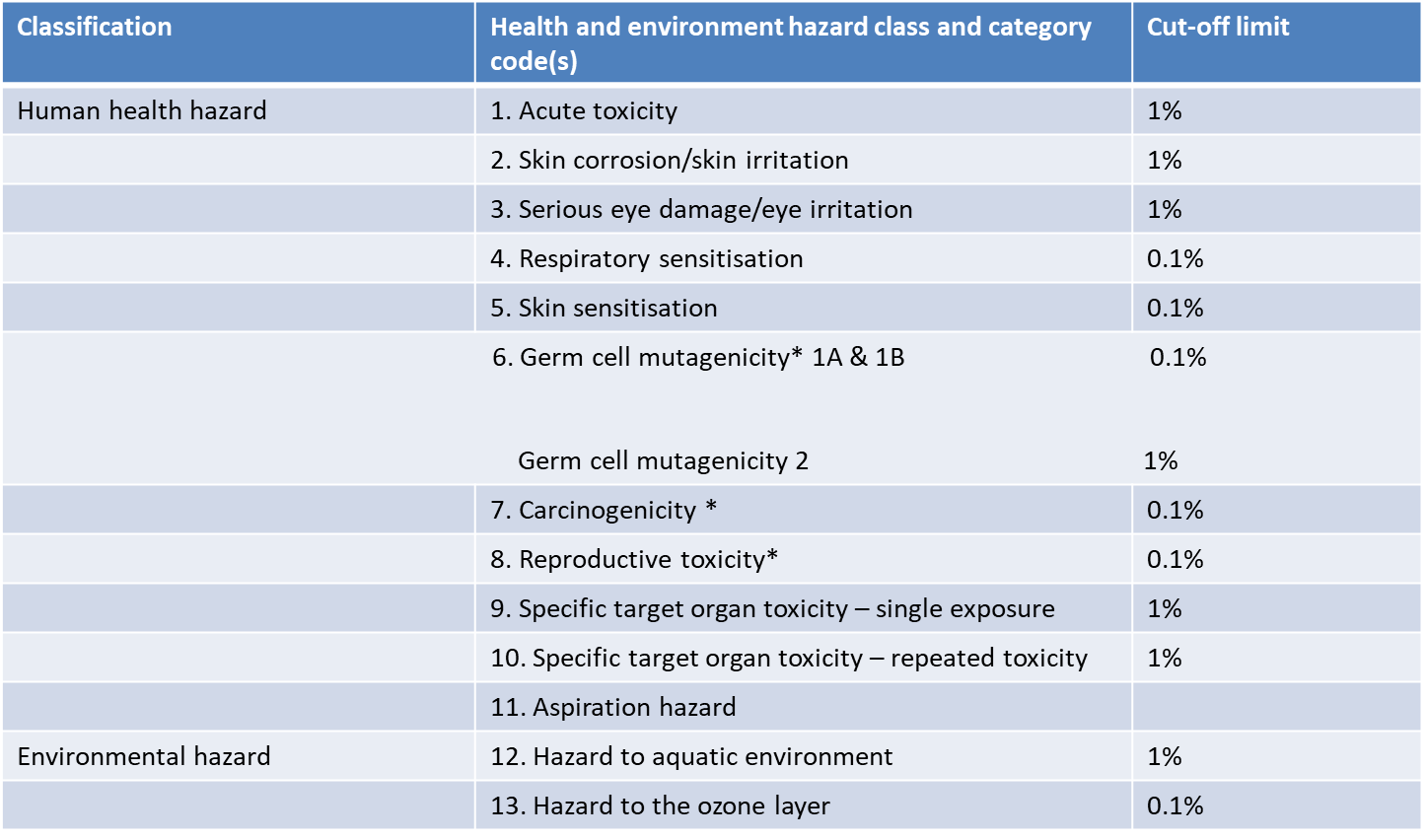
You cannot apply for CBI protection for all ingredients. There are specific cut-off limits for health and environmental hazards, ranging from 0.1 % to 1% across 13 categories. Please see the relevant cut-off limits in the table below.
MoEL Notice No. 2020-130 – Revised Cut-off Values
If the product does not contain hazardous substances, companies can submit a letter of confirmation (LoC), which declares the absence of hazardous substances. And in this way the company would not have to provide 100% composition information. But authorities have the right to question this and require the submission of necessary evidence.
Please note, an LoC is also required under South Korea’s Chemical Control Act (CCA), but do not confuse the two as the information required in each is different.
Labeling
The MoEL suggests a maximum of four pictograms and six precautionary statements on labels. If there are more, the company should choose them based on the level of hazard.
The South Korean importer or supplier information must be included on the label.
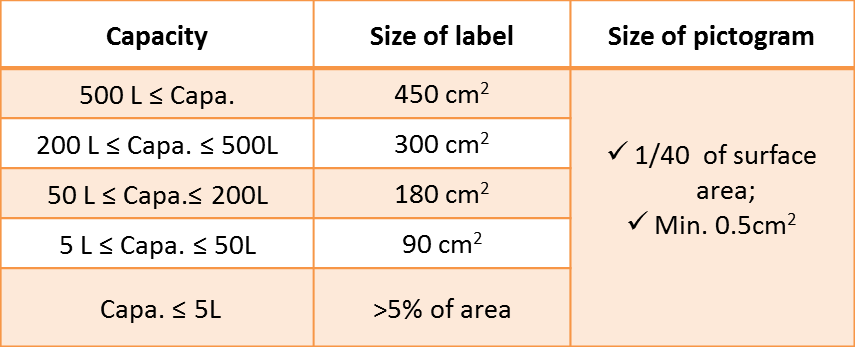
There are also specific label sizes based on volume, a simplified label is applicable for low volumes.
Other things to note
It is also important to note that submission of an MSDS is per product, per importer. So if a foreign company has several importers for one product, they will need to prepare separate Korean MSDSs for each importer and submit the MSDSs separately.
Non-South Korean companies cannot submit an MSDS or apply for CBI protection by themselves. They have two options:
- Ask the South Korean importer to do it on their behalf; or
- Appoint an only representative (OR).
If they choose the first option they will need to provide their importer with 100% composition information.
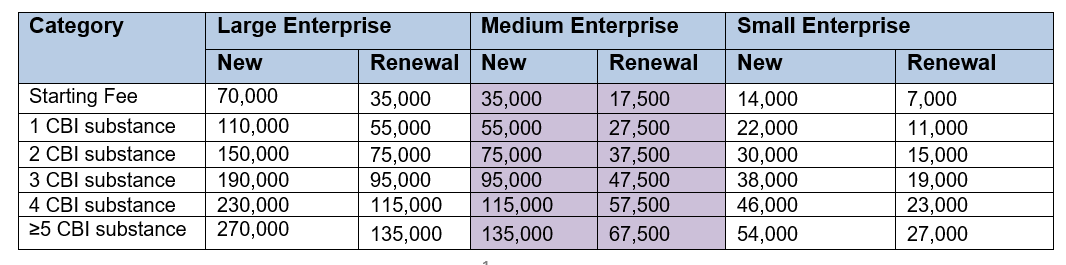
If you appoint an OR to handle your application for CBI protection, the administration fee is based on the size of the OR company that is applying, not the size of the company it is applying on behalf of.
So in the case of the CIRS Group, we are considered a medium size company (see fees below).
Administration fee
- General Substances (KRW)
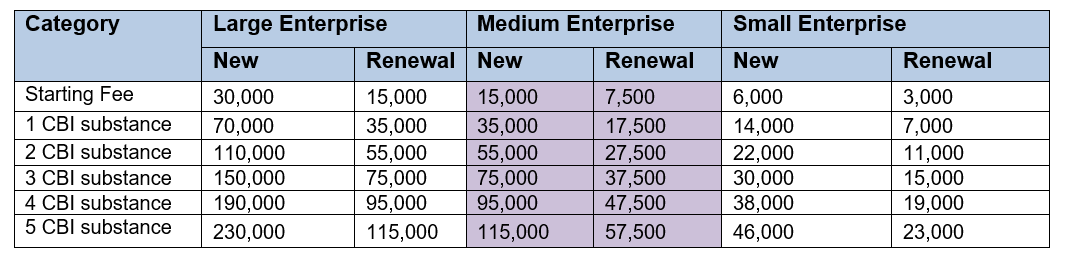
- R&D Substances (KRW)
An OR can only be appointed by the manufacturer or formulator. They fulfil the role of the domestic importer.
The OR could take on the following tasks:
- Preparation and submission of MSDS;
- CBI protection application and extension;
- Rejected application appeal; and
- Delivery of approved MSDS to importers in South Korea.
From our experience, it takes about seven working days to receive approval from the authorities for an MSDS.
Last year, the MoEL also highlighted some areas where they are encountering problems with submitted MSDSs and urged industry to take care when preparing their documents.
This includes:
- Submitting files that have not been translated into Korean;
- Inconsistencies in the information on the MSDSs;
- Not preparing all the required documents;
- Not clearly stating the recommended use(s); and
- Not including detailed information on hazards and risks.

